Chapter 1.2
Atomic building blocks
Introduction
All materials are built up from atoms and molecules, so it is not really surprising that there is a close relationship between the atomic basis of a material and its properties. Important in this context are the nature of the atoms and the ways in which they are arranged. The atoms combine to determine the microstructure of the solid, and, as a consequence, determine its properties. Therefore, if we are to understand the properties of materials, we need to have an understanding of the way atoms can combine to make solids.
Joining atoms together
When two atoms are brought together, they may link to form a molecule; any bonds that form are called primary bonds. Alternatively, they may move apart and so retain their individual identity. Depending on the degree of interaction between the atoms, one of three states can form, these being gases, liquids or solids. These are referred to as the three main phases of matter, where a phase is defined as a structurally homogeneous part of the system and each phase will have its own distinct structure and associated properties. In the gaseous state there is little or no resistance to the relative movement of atoms or molecules, while in the liquid state the resistance to movement is considerably greater, but molecules can still flow past each other with great ease. In solids the movement of atoms and molecules is restricted to a local vibration, although some movement at the atomic level is possible through diffusion.
The controlling factor in bond formation is energy, and a bond will only form if it results in a lowering of the total energy of the atoms being joined. This means that the total energy of the molecule must be less than the sum of the energies of the separate atoms, irrespective of the type of bond being formed. A simple way of visualizing this is the energy-separation diagram, which considers what effect moving two atoms closer together will have on their total energy. A typical energy-separation curve is shown in Figure 1.2.1.
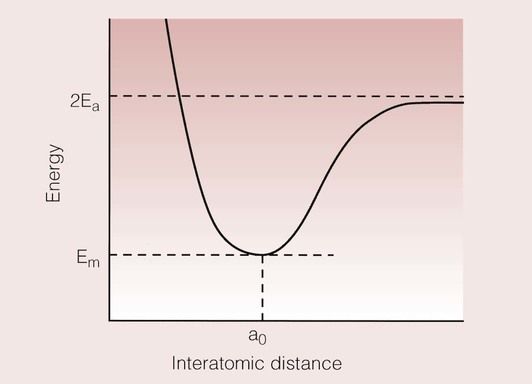
When the two atoms are far apart, the total energy is 2Ea, where Ea is the total energy of one atom. As they are brought closer together, the total energy begins to fall, until it reaches a minimum, Em, at a distance ao. Thereafter, as the atoms are brought more closely together, the total energy increases due to repulsion between their clouds of electrons. As the atoms are brought even closer together, their nuclei begin to repel each other as well, but such proximity is not usually achieved in normal circumstances. Thus, we have attraction at long range, and repulsion at short range.
The conditions under which two atoms will bond together depend on the atoms’ electron configurations, which completely determine their chemical reactivity. The more stable the electron configuration, the less reactive the atom; the extremes of stability are the ‘inert gases’, such as argon, helium and neon, which are almost totally non-reactive. Their near-inertness is caused by their having complete outermost electron orbitals, with no opportunity for more electrons to ‘join’ the atom, and no ‘spare’ or ‘loose’ electrons to leave the atom.
All atoms try to reach their lowest energy state, and this is tantamount to having a complete outermost electron orbital, as the inert gases have. The atoms of some elements have ‘gaps’ for electrons in their outermost orbits, whereas the atoms of other elements have ‘spare’ electrons in their outermost orbits. By combining with each other, these two different types of atoms can both achieve complete outermost orbitals. The formation of bonds, therefore, involves only the outermost valence electrons.
Types of primary bonds
There are three types of primary bond: covalent, ionic and metallic.
Covalent bonds
The covalent bond is the simplest and strongest bond, and arises when atoms share their electrons so that each electron shell achieves an inert gas structure. The formation of such a bond for two hydrogen atoms is shown in Figure 1.2.2.
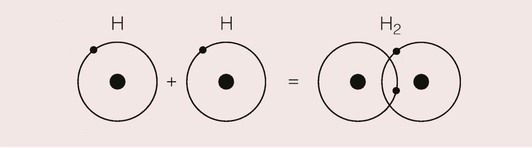
As the two atoms approach one another and the orbitals of the electrons begin to overlap, a molecular orbital is formed where the two electrons are shared between the two nuclei. Since the electrons will spend most of their time in the region where the orbitals overlap, the bond is highly directional.
Ionic bonds
An atom such as sodium would like to lose its single valence electron, as this would give it a configuration similar to that of neon. Naturally, it cannot do so unless there is another atom nearby which will readily accept the electron.
Elements, which can attain an inert gas structure by acquiring a single extra electron, are fluorine, chlorine, bromine and iodine, collectively known as the halogens. Thus, if a sodium and a chlorine atom are allowed to interact, there is a complete transfer of the valence electron from the sodium atom to the chlorine atom. Both attain an inert gas structure, with sodium having a positive charge due to loss of a negative electron, and chlorine a negative charge due to its acquisition of the extra electron. These two ions will be attracted to one another because of their opposite electrical charges, and there is a reduction in the total energy of the pair as they approach. This is shown in the model in Figure 1.2.3; such bonds are called ionic bonds.
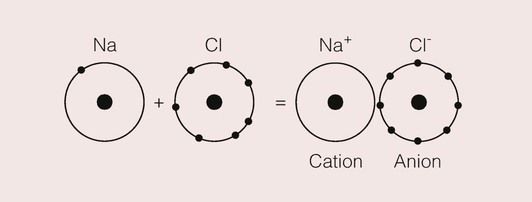
An important difference between the covalent bond and the ionic bond is that the latter is not directional. This is because ionic bonds are a result of the electrostatic fields that surround ions, and these fields will interact with any other ions in the vicinity.
Metallic bonds
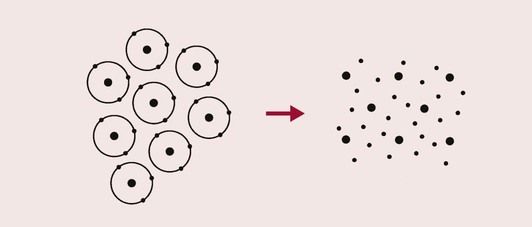
The third primary bond is the metallic bond. It occurs when there is a large aggregate of atoms, usually in a solid, which readily give up the electrons in their valence shells. In such a situation, the electrons can move about quite freely through the solid, spending their time moving from atom to atom. The electron orbitals in the metallic bond have a lower energy than the electron orbitals of the individual atoms. This is because the valence electrons are always closer to one or other nucleus than would be the case in an isolated atom. A cloud of electrons, as shown in Figure 1.2.4, surrounds the atoms. Like the ionic bond, this bond is non-directional.
Bond energies
An important feature of a bond is the bond energy. This is the amount of energy that has to be supplied to separate the two atoms, and is equal to 2Ea − Em, as defined in Figure 1.2.1. Typical bond energies for each of the three types of bond are given in Table 1.2.1.
Table 1.2.1
Typical bond energies for the three bond types
| Atoms bonded | Bond type/>
Only gold members can continue reading. Log In or Register to continue |
Jan 1, 2015 | Posted by in Dental Materials | Comments Off on 1.2: Atomic building blocks
Go to lepin
r/lepin
r/lepin
Unofficial community about Lepin and other alternative bricks and minifigures.
Please read the guides in the sidebar («About» in mobile version).
Members
Online
•
by
tarataqa
I built a Schnauzer (Atomic Building Nanoblocks 6618-1)


More posts you may like
Building a web page using an Atomic Blocks Layout is easy. And, I hope, these Atomic Blocks tips will help you hit the ground running.
- What do the page Layout templates look like?
- How do I build a page using a Layout template?
If you’re new to using the Layout templates, you’ll find these Atomic Blocks tips for layout templates useful.
Don’t just type your text directly into the web page. Save all of your text as a text file using a text editor, such as Word Pad. Then if you want to start over, you won’t lose the text.
My Hero Header image looks offside or funny
If this happens to you, try choosing a different width option for the header block.
If the hero header looks offside in your theme, try a different width option button.
No blog posts
The Freelance Layout template has a section labeled “My latest blog posts”, but no posts are showing.
That’s because you haven’t published any blog posts yet. When you publish blog posts, your most recent blog posts will automatically display here.

Change the Google Map and Mailto: link
The Atomic Blocks Business Layout template has a Google Map, and a button that opens your email program when clicked.
We will be using Google Maps in this tutorial.
1.) Search for your address, post code or zip code.
2.) Click the Share button.
3.) Select the Embed a Map tab.
4.) Then click the “Copy HTML” option.

5.) In the WordPress post editor, click the HTML block. Remove the demo code and paste in your new map code.
Replacing the “Drop us an email!” link
- Click the button to open the edit dialogue box.
- Replace name@example.com with your own email address.
But be careful not to remove the mailto: section.
An example…
mailto:hmqueen@royal.uk
How do I add my Mailchimp API key, so my newsletter signup form will work?
You will need a free Mailchimp account to do this.
First get your API key from Mailchimp.
Then go to Dashboard >Atomic Blocks > Settings: and paste your API key in the box.
You will also need to choose the list you want to use for that specific Newsletter block.
This newsletter signup block comes from the Product Launch One layout.
Click the Configure Your Settings link, and select the list you want to use. The setting is in the WordPress post editor sidebar.

Some of the text is too big!
Some themes use small body text and some themes use large body text. This is especially noticeable when you change from one theme to another.
If you want to reduce the size of paragraph text in a particular block…
1.) Select the block.
2.) In the paragraph’s block settings change the font size.
If you mess things up, you can start again (this is an important Atomic Blocks tip!)
There is an easy way to remove all of the blocks on your page, so you can start that page afresh.
- Click the three dot button in the top right of the WordPress post editor.
- Select the Code Editor option.
- You now see the HTML that makes up this page.
- Select all of the HTML, then delete all of the code.
You are now in the code editor, and you need to go back to the visual editor.
The green bar is hiding the button we need. So click the X close button.
Now you can see, and click the Exit Code Editor Button.
You now have a clean, empty page. You can start afresh.
Looking for a StudioPress theme or hosting?
Check out the two links below…
By Dr.Reham Mohammed Abdallah Items to be covered Types of Atomic Bonding I. Primary bonds Ionic bond Covalent bond Metallic bond II. Secondary bonds Hydrogen bond VanderWaal bond Arrangement of atoms in solids Crystalline solids Non-crystalline solids All materials are built up from atoms and molecules and there is a strong relationship between atomic basis of a material and its properties. To understand the properties of materials, we need to have an understanding of the way atoms can combine to make solids. Types of Atomic Bonding I. Primary bonds 1. Ionic bond It is the attraction of positive and negative ions. The classic example is sodium chloride (Na Cl ) because the sodium atom contains one valence electron in its outer shell and the chlorine atom has seven electrons in its outer shell. The transfer of the sodium valence electron to the chlorine atom results in the stable compound Na Cl. (Fig.1) Ionic materials are characteristically hard and brittle. Electrically and thermally insulators. In dentistry ionic bond is found in gypsum investment, phosphate cement and ceramic. Fig.1: Ionic bond. 2. Covalent bond Sharing of electrons between adjacent atoms. Example: a molecule of methane(CH4). The carbon atom has four valence electrons, whereas each of the four hydrogen atoms has a single valence electron (Fig.2). Covalently bonded materials are typically electrical and thermal insulators. In dentistry: monomer molecules of dental resin are held by covalent bonds within the polymer chain. Fig.2: Covalent bond. 3. Metallic bond “It is the attraction between +ve cores and free electrons or electron cloud”. It occurs in metals, because they easily give up the electrons in their valence shells giving positives cores. The electrons move freely through the metal from atom to atom and from electron cloud. There is attraction between free electrons and the positively charged cores. The metallic bond is weaker than the ionic and the covalent bonds (Fig.2.3). This structure gives the metal: 1. Excellent thermal and electrical conductivity 2. Opacity(due to absorption of light by free electrons). 3. Lustrous appearance. 4.The ability to be plastically deformed. Fig.2.3: Metallic bond. II. Secondary bond In contrast with primary bonds, secondary bonds don’t share electrons. Instead, charge variations among molecules or atomic groups induce polar forces that attract the molecules . These forces are physical, weak and arise from the polarization of molecules i.e. formation of electrical dipoles. 1. Permanent dipole An electrical dipole or polarization exists in a molecule that has an electrical imbalance and hence has a center of positive charge and a center of negative charge. This type of attraction forces occurs in asymmetric molecules where one atom can attract the electrons and becomes negative, and the other atom becomes positive in relation to it resulting in a permanent dipoles. Hydrogen bond The hydrogen bond is an important example. In H2O, there is a covalent bond because oxygen and hydrogen atoms share electrons. However, the electrons around oxygen nucleus are more than those around the hydrogen nucleus and as a result the hydrogen portion of the water molecule is positive in relation to the oxygen portion. Therefore “attraction will take place between the positive hydrogen portion of one water molecule and the negative oxygen portion of another water molecule” (Fig.4). Example: Absorption of water by synthetic resin. Fig.4: Hydrogen bridges formed between water molecules. 2. Van der Waal bond (fluctuating dipole) “it is the attraction between the +ve pole of one atom and the –ve pole of another atom”. In symmetric molecule, such as in inert gases, the electron field is constantly fluctuating. Normally, the electrons of the atoms are distributed equally around the nucleus and produce an electrostatic field around the atom. However, this field may fluctuate and the electrons may concentrate toward one side of the atom and as a result its charge becomes momentarily positive and negative. A fluctuating dipole thus created which will attract other similar dipoles. Such interatomic forces are quite weak (Fig.5). Chemisorption of gases by alloy liquids is followed by attraction due to vander Waal, forces. Fig.5: Fluctuating dipoles. A solid whose molecules are bonded together by Van Der Waal forces has: 1- Low modulus of elasticity. 2- Low melting point. 3- High thermal expansion e.g. waxes, acrylic resin STRUCTURAL ARRANGEMENT OF ATOMS IN SOLIDS All solid materials may be classified on the basis of their atom arrangements as: Crystalline Non crystalline or amorphous Semi crystalline 1. Crystalline Solids Solid dental materials are termed crystalline when their atoms are regularly arranged in a space lattice. A space lattice is the regular arrangement of atoms in the space so that every atom is situated similary to every other atom. (Fig.2.6). Fig.6: Space lattice of crystalline solid material. There are about 14 different types of space lattice but only few are of dental interest. The simplest way to study these types, is to consider a unit cell which is the smallest repeating unit in the space lattice.(fig.7) II. Non-crystalline solids Amorphous means without shape. Gases and liquids are amorphous substances. Some solids like glass and some polymers are amorphous because of the random arrangement of their atoms, yet their atoms may form a short localized range of order lattice with a considerable number of disordered units in between. Since such an arrangement may be considered typical of the liquid structure, these solids are sometimes called “super cooled liquids”(Fig.9). Crystalline solids 1) Have definite Melting temperature. 2) Have regular unit Cell with repetition. Amorphous solids 1) No definite melting Temp (gradually soften on heating and gradually harden on cooling) 2) No regular unit cell But may have a short range of regularity but no repetition.
Many chemists chart the periodic table on a spiral. Most tables
introduce gaps at certain intervals, to account for gaps in the
progression of stable properties along the progress of atomic numbers (Z-numbers, usually equal to electron numbers). Maurice Peyroux’s periodic table introduces gaps from 2He Helium to 3Li Lithium; 10Ne Neon to 11Na Sodium; and 18Ar Argon to 19K Potassium. He extends the fields for 1H Hydrogen, 4Be Beryllium, 12Mg Magnesium, and 21Sc Scandium. Similar adjustments continue among the heavier metals, to align some atomic properties in radial columns.
By making four slight tabulation adjustments in the standard gaps, the Peyroux table core forms a 6×5 squared spiral of elements:
10Ne Neon and 18Ar Argon move across the artificial transition to the left;
11Na Sodium moves into part of the large 12Mg Magnesium field;
18Ar Argon displaces 19K Potassium into the Sc21 Scandium field;
20Ca Calcium moves into part of the 21Sc Scandium field.
Here is Peyroux’s periodic table spiral as a squared grid, with elements over their atomic numbers (number of protons, or number of electrons):………….[tables are omitted in this extract]……….
Elements in the same rows are seven or eight protons apart, thus one electron orbital apart; here marked [v]. Gaps _ appear from 7N to 8 O; and from 15P to 16S. The periodic table compares directly with the stoneprint types: axial opposites are seven or eight types apart; higher magnitudes are fifteen types apart. Two galactic and two polar points intervene in ‘gaps’. Here is Peyroux’s periodic spiral table of elements, over atomic numbers (identical to stoneprint type numbers), over seasons or myths or constellations: ……..[tables are omitted in this extract]…………
Four gaps (marked =) coincide with two galactic and two polar features:
7g Galactic Centre [Galax]
11p Galactic Pole [pGal]
15g Galactic Gate [Gate]; Electrons [e-]?
4p Galactic South Pole [pGs]; NO GAP, but some tables do place a gap between 19K Potassium and 20Ca Calcium.
The four transitions at top and bottom, coincide with four semi-types:
2c Basket; 2He to 3Li, gas to solid
5c Basket Tail; 5:20Ca to 6C, silt to fuel
9c Basket Lid; 9F to 10Ne, reactive to inert gas
13c Basket Head; 13Al to 14Si, metal to rock.
These four structural points, and four semi-types, were first isolated in art and rock art analysis; then confirmed in organ reflexology points; and tabulated by seasons; then confirmed in buildings and site analyses; then tabulated among elements. Thus nature confirms cultural structure. In hindsight, this study should have started with natural structure. However the result is the same: structure is pervasive, enabling creation and perception, including ‘thought’. One of the differences between the cultural stoneprint spiral, and the natural periodic table spiral, is that the atomic spiral is contracted, or more tightly rolled, introducing a coil of ‘opposite’ types between lower and higher magnitude versions of the double-layered types. Here is a comparison ……………….[extract from Stoneprint, 2016]
[order the book Stoneprint, the human code in art, buildings and cities, at $30 plus postage from Four Equators Media, via edmondfurter at gmail dot com].












