WARNINGS
Included as part of the PRECAUTIONS section.
PRECAUTIONS
Use Of QUALAQUIN For Treatment Or Prevention Of Nocturnal Leg Cramps
QUALAQUIN may cause unpredictable serious and life-threatening hematologic reactions including thrombocytopenia and hemolytic-uremic syndrome/thrombotic thrombocytopenic purpura (HUS/TTP) in addition to hypersensitivity reactions, QT prolongation, serious cardiac arrhythmias including torsades de pointes, and other serious adverse events requiring medical intervention and hospitalization. Chronic renal impairment associated with the development of TTP, and fatalities have also been reported. The risk associated with the use of QUALAQUIN in the absence of evidence of its effectiveness for treatment or prevention of nocturnal leg cramps, outweighs any potential benefit in treating and/or preventing this benign, self-limiting condition.
Thrombocytopenia
Quinine-induced thrombocytopenia is an immune-mediated disorder. Severe cases of thrombocytopenia that are fatal or life threatening have been reported, including cases of HUS/TTP. Chronic renal impairment associated with the development of TTP has also been reported. Thrombocytopenia usually resolves within a week upon discontinuation of quinine. If quinine is not stopped, a patient is at risk for fatal hemorrhage. Upon re-exposure to quinine from any source, a patient with quinine-dependent antibodies could develop thrombocytopenia that is more rapid in onset and more severe than the original episode.
QT Prolongation And Ventricular Arrhythmias
QT interval prolongation has been a consistent finding in studies which evaluated electrocardiographic changes with oral or parenteral quinine administration, regardless of age, clinical status, or severity of disease. The maximum increase in QT interval has been shown to correspond with peak quinine plasma concentration. Quinine sulfate has been rarely associated with potentially fatal cardiac arrhythmias, including torsades de pointes, and ventricular fibrillation.
QUALAQUIN has been shown to cause concentration-dependent prolongation of the PR and QRS interval. At particular risk are patients with underlying structural heart disease and preexisting conduction system abnormalities, elderly patients with sick sinus syndrome, patients with atrial fibrillation with slow ventricular response, patients with myocardial ischemia or patients receiving drugs known to prolong the PR interval (e.g. verapamil) or QRS interval (e.g. flecainide or quinidine).
QUALAQUIN is not recommended for use with other drugs known to cause QT prolongation, including Class IA antiarrhythmic agents (e.g., quinidine, procainamide, disopyramide), and Class III antiarrhythmic agents (e.g., amiodarone, sotalol, dofetilide).
The use of macrolide antibiotics such as erythromycin should be avoided in patients receiving QUALAQUIN. Fatal torsades de pointes was reported in an elderly patient who received concomitant quinine, erythromycin, and dopamine. Although a causal relationship between a specific drug and the arrhythmia was not established in this case, erythromycin is a CYP3A4 inhibitor and has been shown to increase quinine plasma levels when used concomitantly. A related macrolide antibiotic, troleandomycin, has also been shown to increase quinine exposure in a pharmacokinetic study.
Quinine may inhibit the metabolism of certain drugs that are CYP3A4 substrates and are known to cause QT prolongation, e.g., astemizole, cisapride, terfenadine, pimozide, halofantrine and quinidine. Torsades de pointes has been reported in patients who received concomitant quinine and astemizole. Therefore, concurrent use of QUALAQUIN with these medications, or drugs with similar properties, should be avoided.
Concomitant administration of QUALAQUIN with the antimalarial drugs, mefloquine or halofantrine, may result in electrocardiographic abnormalities, including QT prolongation, and increase the risk for torsades de pointes or other serious ventricular arrhythmias. Concurrent use of QUALAQUIN and mefloquine may also increase the risk of seizures.
QUALAQUIN should also be avoided in patients with known prolongation of QT interval and in patients with clinical conditions known to prolong the QT interval, such as uncorrected hypokalemia, bradycardia, and certain cardiac conditions.
Concomitant Use Of Rifampin
Treatment failures may result from the concurrent use of rifampin with QUALAQUIN, due to decreased plasma concentrations of quinine, and concomitant use of these medications should be avoided.
Concomitant Use Of Neuromuscular Blocking Agents
The use of neuromuscular blocking agents should be avoided in patients receiving QUALAQUIN. In one patient who received pancuronium during an operative procedure, subsequent administration of quinine resulted in respiratory depression and apnea. Although there are no clinical reports with succinylcholine or tubocurarine, quinine may also potentiate neuromuscular blockade when used with these drugs.
Hypersensitivity
Serious hypersensitivity reactions reported with quinine sulfate include anaphylactic shock, anaphylactoid reactions, urticaria, serious skin rashes, including Stevens-Johnson syndrome and toxic epidermal necrolysis, angioedema, facial edema, bronchospasm, and pruritus.
A number of other serious adverse reactions reported with quinine, including thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS), thrombocytopenia, immune thrombocytopenic purpura (ITP), blackwater fever, disseminated intravascular coagulation, leukopenia, neutropenia, granulomatous hepatitis, and acute interstitial nephritis may also be due to hypersensitivity reactions.
QUALAQUIN should be discontinued in case of any signs or symptoms of hypersensitivity.
Atrial Fibrillation And Flutter
QUALAQUIN should be used with caution in patients with atrial fibrillation or atrial flutter. A paradoxical increase in ventricular response rate may occur with quinine, similar to that observed with quinidine. If digoxin is used to prevent a rapid ventricular response, serum digoxin levels should be closely monitored, because digoxin levels may be increased with use of quinine.
Hypoglycemia
Quinine stimulates release of insulin from the pancreas, and patients, especially pregnant women, may experience clinically significant hypoglycemia.
Patient Counseling Information
See FDA-approved patient labeling (Medication Guide)
Dosing Instructions
Patients should be instructed to:
- Take all of the medication as directed.
- Take no more of the medication than the amount prescribed.
- Take with food to minimize possible gastrointestinal irritation.
If a dose is missed, patients should also be instructed not to double the next dose. If more than 4 hours has elapsed since the missed dose, the patient should wait and take the next dose as previously scheduled.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment Of Fertility
Carcinogenesis
Carcinogenicity studies of quinine have not been conducted.
Mutagenesis
Genotoxicity studies of quinine were positive in the Ames bacterial mutation assay with metabolic activation and in the sister chromatid exchange assay in mice. The sex-linked recessive lethal test performed in Drosophila, the in vivo mouse micronucleus assay, and the chromosomal aberration assay in mice and Chinese hamsters were negative.
Impairment of Fertility
Published studies indicate that quinine produces testicular toxicity in mice at a single intraperitoneal dose of 300 mg/kg corresponding to a dose of approximately 0.75 times the maximum recommended human dose (MRHD; 32 mg/kg/day) and in rats at an intramuscular dose of 10 mg/kg/day, 5 days/week, for 8 weeks corresponding to a daily dose of approximately 0.05 times the MRHD based on body surface area (BSA) comparisons. The findings include atrophy or degeneration of the seminiferous tubules, decreased sperm count and motility, and decreased testosterone levels in the serum and testes. There was no effect on testes weight in studies of oral doses of up to 500 mg/kg/day in mice and 700 mg/kg/day in rats (approximately 1.2 and 3.5 times the MRHD respectively based on BSA comparisons). In a published study in 5 men receiving 600 mg of quinine TID for one week, sperm motility was decreased and percent sperm with abnormal morphology was increased; sperm count and serum testosterone were unaffected.
Use In Specific Populations
Pregnancy
Pregnancy Category C
There are extensive published data but few well-controlled studies of QUALAQUIN in pregnant women. Published data on over 1,000 pregnancy exposures to quinine did not show an increase in teratogenic effects over the background rate in the general population; however, the majority of these exposures were not in the first trimester. In developmental and reproductive toxicity studies, central nervous system (CNS) and ear abnormalities and increased fetal deaths occurred in some species when pregnant animals received quinine at doses about 1 to 4 times the human clinical dose. Quinine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
P. falciparum malaria carries a higher risk of morbidity and mortality in pregnant women than in the general population. Pregnant women with P. falciparum malaria have an increased incidence of fetal loss (including spontaneous abortion and stillbirth), preterm labor and delivery, intrauterine growth retardation, low birth weight, and maternal death. Therefore, treatment of malaria in pregnancy is important.
Hypoglycemia, due to increased pancreatic secretion of insulin, has been associated with quinine use, particularly in pregnant women.
Quinine crosses the placenta with measurable blood concentrations in the fetus. In 8 women who delivered live infants 1 to 6 days after starting quinine therapy, umbilical cord plasma quinine concentrations were between 1.0 and 4.6 mg/L (mean 2.4 mg/L) and the mean (±SD) ratio of cord plasma to maternal plasma quinine concentrations was 0.32 ± 0.14. Quinine levels in the fetus may not be therapeutic. If congenital malaria is suspected after delivery, the infant should be evaluated and treated appropriately.
A study from Thailand (1999) of women with P. falciparum malaria who were treated with oral quinine sulfate 10 mg/kg 3 times daily for 7 days at anytime in pregnancy reported no significant difference in the rate of stillbirths at > 28 weeks of gestation in women treated with quinine (10 of 633 women [1.6%]) as compared with a control group without malaria or exposure to antimalarial drugs during pregnancy (40 of 2201 women [1.8%]). The overall rate of congenital malformations (9 of 633 offspring [1.4%]) was not different for women who were treated with quinine sulfate compared with the control group (38 of 2201 offspring [1.7%]). The spontaneous abortion rate was higher in the control group (10.9%) than in women treated with quinine sulfate (3.5%) [OR = 3.1; 95% CI 2.1-4.7]. An epidemiologic survey that included 104 mother-child pairs exposed to quinine during the first 4 months of pregnancy, found no increased risk of structural birth defects was seen (2 fetal malformations [1.9%]). Rare and isolated case reports describe deafness and optic nerve hypoplasia in children exposed in utero due to maternal ingestion of high doses of quinine.
In animal developmental studies conducted in multiple animal species, pregnant animals received quinine by the subcutaneous or intramuscular route at dose levels similar to the maximum recommended human dose (MRHD; 32 mg/kg/day) based on body surface area (BSA) comparisons. There were increases in fetal death in utero in rabbits at maternal doses ≥ 100 mg/kg/day and in dogs at ≥ 15 mg/kg/day corresponding to dose levels approximately 0.5 and 0.25 times the MRHD respectively based on BSA comparisons. Rabbit offspring had increased rates of degenerated auditory nerve and spiral ganglion and increased rates of CNS anomalies such as anencephaly and microcephaly at a dose of 130 mg/kg/day corresponding to a maternal dose approximately 1.3 times the MRHD based on BSA comparison. Guinea pig offspring had increased rates of hemorrhage and mitochondrial change in the cochlea at maternal doses of 200 mg/kg corresponding to a dose level of approximately 1.4 times the MRHD based on BSA comparison. There were no teratogenic findings in rats at maternal doses up to 300 mg/kg/day and in monkeys at doses up to 200 mg/kg/day corresponding to doses approximately 1 and 2 times the MRHD respectively based on BSA comparisons.
In a pre-postnatal study in rats, an estimated oral dose of quinine sulfate of 20 mg/kg/day corresponding to approximately 0.1 times the MRHD based on BSA comparison resulted in offspring with impaired growth, lower body weights at birth and during the lactation period, and delayed physical development of teeth eruption and eye opening during the lactation period.
Labor And Delivery
There is no evidence that quinine causes uterine contractions at the doses recommended for the treatment of malaria. In doses several-times higher than those used to treat malaria, quinine may stimulate the pregnant uterus.
Nursing Mothers
There is limited information on the safety of quinine in breastfed infants. No toxicity was reported in infants in a single study where oral quinine sulfate (10 mg/kg every 8 hours for 1 to 10 days) was administered to 25 lactating women. It is estimated from this study that breastfed infants would receive less than 2 to 3 mg per day of quinine base ( < 0.4% of the maternal dose) via breast milk.
Although quinine is generally considered compatible with breastfeeding, the risks and benefits to infant and mother should be assessed. Caution should be exercised when administered to a nursing woman.
If malaria is suspected in the infant, appropriate evaluation and treatment should be provided. Plasma quinine levels may not be therapeutic in infants of nursing mothers receiving QUALAQUIN.
Pediatric Use
The safety and efficacy of QUALAQUIN in pediatric patients under the age of 16 has not been established.
Geriatric Use
Clinical studies of quinine sulfate did not include sufficient numbers of subjects aged 65 and over to determine whether they respond to treatment differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
Renal Impairment
Clearance of quinine is decreased in patients with severe chronic renal failure. The dosage and dosing frequency should be reduced.
Hepatic Impairment
In patients with severe hepatic impairment (Child-Pugh C), quinine oral clearance (CL/F) is decreased, volume of distribution (Vd/F) is increased, and half-life is prolonged, relative to subjects with normal liver function. Therefore, quinine is not indicated in patients with severe hepatic impairment and alternate therapy should be administered.
Close monitoring is recommended for patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment, as exposure to quinine may be increased relative to subjects with normal liver function.
WARNINGS
Included as part of the PRECAUTIONS section.
PRECAUTIONS
Use Of QUALAQUIN For Treatment Or Prevention Of Nocturnal Leg Cramps
QUALAQUIN may cause unpredictable serious and life-threatening hematologic reactions including thrombocytopenia and hemolytic-uremic syndrome/thrombotic thrombocytopenic purpura (HUS/TTP) in addition to hypersensitivity reactions, QT prolongation, serious cardiac arrhythmias including torsades de pointes, and other serious adverse events requiring medical intervention and hospitalization. Chronic renal impairment associated with the development of TTP, and fatalities have also been reported. The risk associated with the use of QUALAQUIN in the absence of evidence of its effectiveness for treatment or prevention of nocturnal leg cramps, outweighs any potential benefit in treating and/or preventing this benign, self-limiting condition.
Thrombocytopenia
Quinine-induced thrombocytopenia is an immune-mediated disorder. Severe cases of thrombocytopenia that are fatal or life threatening have been reported, including cases of HUS/TTP. Chronic renal impairment associated with the development of TTP has also been reported. Thrombocytopenia usually resolves within a week upon discontinuation of quinine. If quinine is not stopped, a patient is at risk for fatal hemorrhage. Upon re-exposure to quinine from any source, a patient with quinine-dependent antibodies could develop thrombocytopenia that is more rapid in onset and more severe than the original episode.
QT Prolongation And Ventricular Arrhythmias
QT interval prolongation has been a consistent finding in studies which evaluated electrocardiographic changes with oral or parenteral quinine administration, regardless of age, clinical status, or severity of disease. The maximum increase in QT interval has been shown to correspond with peak quinine plasma concentration. Quinine sulfate has been rarely associated with potentially fatal cardiac arrhythmias, including torsades de pointes, and ventricular fibrillation.
QUALAQUIN has been shown to cause concentration-dependent prolongation of the PR and QRS interval. At particular risk are patients with underlying structural heart disease and preexisting conduction system abnormalities, elderly patients with sick sinus syndrome, patients with atrial fibrillation with slow ventricular response, patients with myocardial ischemia or patients receiving drugs known to prolong the PR interval (e.g. verapamil) or QRS interval (e.g. flecainide or quinidine).
QUALAQUIN is not recommended for use with other drugs known to cause QT prolongation, including Class IA antiarrhythmic agents (e.g., quinidine, procainamide, disopyramide), and Class III antiarrhythmic agents (e.g., amiodarone, sotalol, dofetilide).
The use of macrolide antibiotics such as erythromycin should be avoided in patients receiving QUALAQUIN. Fatal torsades de pointes was reported in an elderly patient who received concomitant quinine, erythromycin, and dopamine. Although a causal relationship between a specific drug and the arrhythmia was not established in this case, erythromycin is a CYP3A4 inhibitor and has been shown to increase quinine plasma levels when used concomitantly. A related macrolide antibiotic, troleandomycin, has also been shown to increase quinine exposure in a pharmacokinetic study.
Quinine may inhibit the metabolism of certain drugs that are CYP3A4 substrates and are known to cause QT prolongation, e.g., astemizole, cisapride, terfenadine, pimozide, halofantrine and quinidine. Torsades de pointes has been reported in patients who received concomitant quinine and astemizole. Therefore, concurrent use of QUALAQUIN with these medications, or drugs with similar properties, should be avoided.
Concomitant administration of QUALAQUIN with the antimalarial drugs, mefloquine or halofantrine, may result in electrocardiographic abnormalities, including QT prolongation, and increase the risk for torsades de pointes or other serious ventricular arrhythmias. Concurrent use of QUALAQUIN and mefloquine may also increase the risk of seizures.
QUALAQUIN should also be avoided in patients with known prolongation of QT interval and in patients with clinical conditions known to prolong the QT interval, such as uncorrected hypokalemia, bradycardia, and certain cardiac conditions.
Concomitant Use Of Rifampin
Treatment failures may result from the concurrent use of rifampin with QUALAQUIN, due to decreased plasma concentrations of quinine, and concomitant use of these medications should be avoided.
Concomitant Use Of Neuromuscular Blocking Agents
The use of neuromuscular blocking agents should be avoided in patients receiving QUALAQUIN. In one patient who received pancuronium during an operative procedure, subsequent administration of quinine resulted in respiratory depression and apnea. Although there are no clinical reports with succinylcholine or tubocurarine, quinine may also potentiate neuromuscular blockade when used with these drugs.
Hypersensitivity
Serious hypersensitivity reactions reported with quinine sulfate include anaphylactic shock, anaphylactoid reactions, urticaria, serious skin rashes, including Stevens-Johnson syndrome and toxic epidermal necrolysis, angioedema, facial edema, bronchospasm, and pruritus.
A number of other serious adverse reactions reported with quinine, including thrombotic thrombocytopenic purpura (TTP) and hemolytic uremic syndrome (HUS), thrombocytopenia, immune thrombocytopenic purpura (ITP), blackwater fever, disseminated intravascular coagulation, leukopenia, neutropenia, granulomatous hepatitis, and acute interstitial nephritis may also be due to hypersensitivity reactions.
QUALAQUIN should be discontinued in case of any signs or symptoms of hypersensitivity.
Atrial Fibrillation And Flutter
QUALAQUIN should be used with caution in patients with atrial fibrillation or atrial flutter. A paradoxical increase in ventricular response rate may occur with quinine, similar to that observed with quinidine. If digoxin is used to prevent a rapid ventricular response, serum digoxin levels should be closely monitored, because digoxin levels may be increased with use of quinine.
Hypoglycemia
Quinine stimulates release of insulin from the pancreas, and patients, especially pregnant women, may experience clinically significant hypoglycemia.
Patient Counseling Information
See FDA-approved patient labeling (Medication Guide)
Dosing Instructions
Patients should be instructed to:
- Take all of the medication as directed.
- Take no more of the medication than the amount prescribed.
- Take with food to minimize possible gastrointestinal irritation.
If a dose is missed, patients should also be instructed not to double the next dose. If more than 4 hours has elapsed since the missed dose, the patient should wait and take the next dose as previously scheduled.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment Of Fertility
Carcinogenesis
Carcinogenicity studies of quinine have not been conducted.
Mutagenesis
Genotoxicity studies of quinine were positive in the Ames bacterial mutation assay with metabolic activation and in the sister chromatid exchange assay in mice. The sex-linked recessive lethal test performed in Drosophila, the in vivo mouse micronucleus assay, and the chromosomal aberration assay in mice and Chinese hamsters were negative.
Impairment of Fertility
Published studies indicate that quinine produces testicular toxicity in mice at a single intraperitoneal dose of 300 mg/kg corresponding to a dose of approximately 0.75 times the maximum recommended human dose (MRHD; 32 mg/kg/day) and in rats at an intramuscular dose of 10 mg/kg/day, 5 days/week, for 8 weeks corresponding to a daily dose of approximately 0.05 times the MRHD based on body surface area (BSA) comparisons. The findings include atrophy or degeneration of the seminiferous tubules, decreased sperm count and motility, and decreased testosterone levels in the serum and testes. There was no effect on testes weight in studies of oral doses of up to 500 mg/kg/day in mice and 700 mg/kg/day in rats (approximately 1.2 and 3.5 times the MRHD respectively based on BSA comparisons). In a published study in 5 men receiving 600 mg of quinine TID for one week, sperm motility was decreased and percent sperm with abnormal morphology was increased; sperm count and serum testosterone were unaffected.
Use In Specific Populations
Pregnancy
Pregnancy Category C
There are extensive published data but few well-controlled studies of QUALAQUIN in pregnant women. Published data on over 1,000 pregnancy exposures to quinine did not show an increase in teratogenic effects over the background rate in the general population; however, the majority of these exposures were not in the first trimester. In developmental and reproductive toxicity studies, central nervous system (CNS) and ear abnormalities and increased fetal deaths occurred in some species when pregnant animals received quinine at doses about 1 to 4 times the human clinical dose. Quinine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
P. falciparum malaria carries a higher risk of morbidity and mortality in pregnant women than in the general population. Pregnant women with P. falciparum malaria have an increased incidence of fetal loss (including spontaneous abortion and stillbirth), preterm labor and delivery, intrauterine growth retardation, low birth weight, and maternal death. Therefore, treatment of malaria in pregnancy is important.
Hypoglycemia, due to increased pancreatic secretion of insulin, has been associated with quinine use, particularly in pregnant women.
Quinine crosses the placenta with measurable blood concentrations in the fetus. In 8 women who delivered live infants 1 to 6 days after starting quinine therapy, umbilical cord plasma quinine concentrations were between 1.0 and 4.6 mg/L (mean 2.4 mg/L) and the mean (±SD) ratio of cord plasma to maternal plasma quinine concentrations was 0.32 ± 0.14. Quinine levels in the fetus may not be therapeutic. If congenital malaria is suspected after delivery, the infant should be evaluated and treated appropriately.
A study from Thailand (1999) of women with P. falciparum malaria who were treated with oral quinine sulfate 10 mg/kg 3 times daily for 7 days at anytime in pregnancy reported no significant difference in the rate of stillbirths at > 28 weeks of gestation in women treated with quinine (10 of 633 women [1.6%]) as compared with a control group without malaria or exposure to antimalarial drugs during pregnancy (40 of 2201 women [1.8%]). The overall rate of congenital malformations (9 of 633 offspring [1.4%]) was not different for women who were treated with quinine sulfate compared with the control group (38 of 2201 offspring [1.7%]). The spontaneous abortion rate was higher in the control group (10.9%) than in women treated with quinine sulfate (3.5%) [OR = 3.1; 95% CI 2.1-4.7]. An epidemiologic survey that included 104 mother-child pairs exposed to quinine during the first 4 months of pregnancy, found no increased risk of structural birth defects was seen (2 fetal malformations [1.9%]). Rare and isolated case reports describe deafness and optic nerve hypoplasia in children exposed in utero due to maternal ingestion of high doses of quinine.
In animal developmental studies conducted in multiple animal species, pregnant animals received quinine by the subcutaneous or intramuscular route at dose levels similar to the maximum recommended human dose (MRHD; 32 mg/kg/day) based on body surface area (BSA) comparisons. There were increases in fetal death in utero in rabbits at maternal doses ≥ 100 mg/kg/day and in dogs at ≥ 15 mg/kg/day corresponding to dose levels approximately 0.5 and 0.25 times the MRHD respectively based on BSA comparisons. Rabbit offspring had increased rates of degenerated auditory nerve and spiral ganglion and increased rates of CNS anomalies such as anencephaly and microcephaly at a dose of 130 mg/kg/day corresponding to a maternal dose approximately 1.3 times the MRHD based on BSA comparison. Guinea pig offspring had increased rates of hemorrhage and mitochondrial change in the cochlea at maternal doses of 200 mg/kg corresponding to a dose level of approximately 1.4 times the MRHD based on BSA comparison. There were no teratogenic findings in rats at maternal doses up to 300 mg/kg/day and in monkeys at doses up to 200 mg/kg/day corresponding to doses approximately 1 and 2 times the MRHD respectively based on BSA comparisons.
In a pre-postnatal study in rats, an estimated oral dose of quinine sulfate of 20 mg/kg/day corresponding to approximately 0.1 times the MRHD based on BSA comparison resulted in offspring with impaired growth, lower body weights at birth and during the lactation period, and delayed physical development of teeth eruption and eye opening during the lactation period.
Labor And Delivery
There is no evidence that quinine causes uterine contractions at the doses recommended for the treatment of malaria. In doses several-times higher than those used to treat malaria, quinine may stimulate the pregnant uterus.
Nursing Mothers
There is limited information on the safety of quinine in breastfed infants. No toxicity was reported in infants in a single study where oral quinine sulfate (10 mg/kg every 8 hours for 1 to 10 days) was administered to 25 lactating women. It is estimated from this study that breastfed infants would receive less than 2 to 3 mg per day of quinine base ( < 0.4% of the maternal dose) via breast milk.
Although quinine is generally considered compatible with breastfeeding, the risks and benefits to infant and mother should be assessed. Caution should be exercised when administered to a nursing woman.
If malaria is suspected in the infant, appropriate evaluation and treatment should be provided. Plasma quinine levels may not be therapeutic in infants of nursing mothers receiving QUALAQUIN.
Pediatric Use
The safety and efficacy of QUALAQUIN in pediatric patients under the age of 16 has not been established.
Geriatric Use
Clinical studies of quinine sulfate did not include sufficient numbers of subjects aged 65 and over to determine whether they respond to treatment differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
Renal Impairment
Clearance of quinine is decreased in patients with severe chronic renal failure. The dosage and dosing frequency should be reduced.
Hepatic Impairment
In patients with severe hepatic impairment (Child-Pugh C), quinine oral clearance (CL/F) is decreased, volume of distribution (Vd/F) is increased, and half-life is prolonged, relative to subjects with normal liver function. Therefore, quinine is not indicated in patients with severe hepatic impairment and alternate therapy should be administered.
Close monitoring is recommended for patients with mild (Child-Pugh A) or moderate (Child-Pugh B) hepatic impairment, as exposure to quinine may be increased relative to subjects with normal liver function.
Описание препарата Лимонтар® (таблетки растворимые, 50 мг+200 мг) основано на официальной инструкции, утверждено компанией-производителем в 2014 году
Дата согласования: 10.04.2014
Особые отметки:
Содержание
- Фотографии упаковок
- Действующее вещество
- ATX
- Фармакологическая группа
- Нозологическая классификация (МКБ-10)
- Состав
- Описание лекарственной формы
- Фармакологическое действие
- Фармакодинамика
- Фармакокинетика
- Показания
- Противопоказания
- Способ применения и дозы
- Побочные действия
- Взаимодействие
- Форма выпуска
- Производитель
- Условия отпуска из аптек
- Условия хранения
- Срок годности
- Заказ в аптеках Москвы
- Отзывы
Фотографии упаковок
10.04.2014
Действующее вещество
ATX
Фармакологическая группа
Нозологическая классификация (МКБ-10)
Список кодов МКБ-10
- F06.6 Органическое эмоционально лабильное [астеническое] расстройство
- F10.0 Острая интоксикация алкоголем
- F10.2 Синдром алкогольной зависимости
- F10.3 Абстинентное состояние
- G90 Расстройства вегетативной [автономной] нервной системы
- K94* Диагностика заболеваний ЖКТ
- O26.2 Медицинская помощь женщине с привычным невынашиванием беременности
- P05 Замедленный рост и недостаточность питания плода
- P20 Внутриутробная гипоксия
- R53 Недомогание и утомляемость
- R63.0 Анорексия
- T51 Токсическое действие алкоголя
- Z73.0 Переутомление
- Z73.6 Ограничения деятельности, вызванные снижением трудоспособности
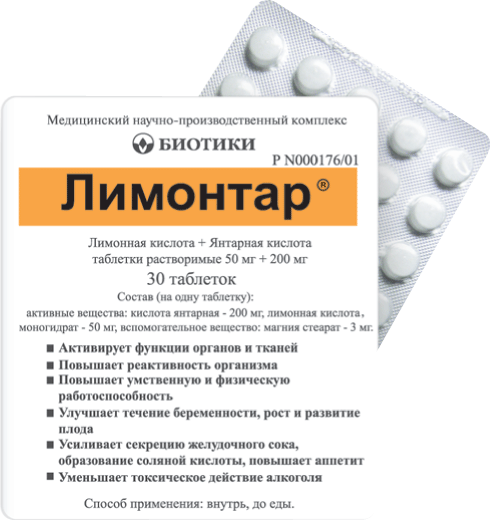
Состав
| Таблетки растворимые | 1 табл. |
| активные вещества: | |
| кислота янтарная | 200 мг |
| лимонная кислота, моногидрат | 50 мг |
| вспомогательные вещества: магния стеарат — 3 мг |
Описание лекарственной формы
Таблетки белого цвета с элементами мраморности, двояковыпуклой формы.
Фармакологическое действие
Фармакологическое действие
—
метаболическое, антиоксидантное, антиалкогольное.
Фармакодинамика
Лимонтар® является регулятором тканевого обмена, усиливает окислительно-восстановительные процессы, образование АТФ, чем обусловлены его антигипоксические и антиоксидантные свойства, и способность препарата:
— активировать функции органов и тканей;
— повышать реактивность организма;
— повышать умственную и физическую работоспособность;
— улучшать течение беременности, рост и развитие плода;
— усиливать секрецию желудочного сока, образование соляной кислоты, повышать аппетит;
— уменьшать токсическое действие алкоголя.
Действие препарата Лимонтар® проявляется через 10–20 мин после приема внутрь.
Фармакокинетика
Янтарная и лимонная кислоты полностью метаболизируются до воды и углекислого газа, накопление в организме не происходит.
Показания
повышение неспецифической реактивности организма беременных женщин, улучшение адаптационных и компенсаторно-защитных возможностей в целях профилактики осложнений при гипоксии и гипотрофии плода, при невынашивании беременности;
профилактика опьянения;
уменьшение токсического влияния алкоголя при остром алкогольном опьянении легкой и средней тяжести и постинтоксикационных расстройствах;
комплексная терапия запойных состояний у больных хроническим алкоголизмом и астеновегетативных расстройств (выраженная общая слабость, снижение работоспособности, аппетита) в период алкогольного абстинентного синдрома;
в качестве «пробного завтрака» при исследовании секреторной и кислотообразующей функции желудка.
Противопоказания
гиперчувствительность;
артериальная гипертензия;
ишемическая болезнь сердца (в т.ч. стенокардия);
глаукома;
язвенная болезнь желудка и двенадцатиперстной кишки в стадии обострения;
поздний гестоз (тяжелая форма).
Способ применения и дозы
Внутрь, до еды. Перед приемом таблетку измельчают и растворяют в воде с питьевой содой (сода — на кончике ножа). Для растворения можно использовать минеральную воду.
Беременным Лимонтар® назначают по 1 табл. в день в течение 10 дней в I триместре (на сроке беременности 12–14 нед) и во II триместре (срок беременности 24–26 нед). В III триместре назначают за 10–25 дней до родов. Общая доза препарата Лимонтар® за период беременности — 5–7,5 г.
В качестве средства для профилактики опьянения — 1 табл. препарата Лимонтар® за 20–60 мин до приема алкоголя.
В состоянии острого алкогольного опьянения препарат назначают по 1 табл. 2–4 раза в сутки с интервалом в 1–2,5 ч.
При купировании запойных состояний Лимонтар® назначают по 1 табл. 3–4 раза в сутки в течение 4–10 дней, как самостоятельно, так и в комплексе с традиционными ЛС.
При назначении в качестве «пробного завтрака» для исследования секреторной и кислотообразующей функции желудка принимают внутрь, натощак 1 табл., предварительно растворив в 10–15 мл воды.
При появлении чувства тяжести в подложечной области, Лимонтар® назначают после еды.
Побочные действия
Возможно появление болей в подложечной области (обычно эти явления проходят самостоятельно через 3–5 мин), повышенная секреция желудочного сока. У лиц, склонных к артериальной гипертензии, после приема препарата может повышаться АД.
Взаимодействие
Ослабляет действие снотворных ЛС, транквилизаторов.
Форма выпуска
Таблетки растворимые, 50 мг + 200 мг. По 30 табл. в контурной ячейковой упаковке, в пачке картонной 1 упаковка.
Производитель
ООО «Медицинский научно-производственный комплекс «БИОТИКИ». Россия, 115404, Москва, ул. 6-я Радиальная, 24, стр.14.
Тел.: + 7(495)327-53-53.
Условия отпуска из аптек
Без рецепта.
Условия хранения
При температуре не выше 25 °C, в оригинальной упаковке (контурная ячейковая упаковка в пачке).
Хранить в недоступном для детей месте.
Срок годности
3 года.
Не применять по истечении срока годности, указанного на упаковке.
Представленная информация о ценах на препараты не является предложением о продаже или покупке товара.
Информация предназначена исключительно для сравнения цен в стационарных аптеках, осуществляющих деятельность в
соответствии со статьей 55 Федерального закона «Об обращении лекарственных средств» от 12.04.2010 № 61-ФЗ.
Форма выпуска, упаковка и состав
препарата Лимонтар®
Таблетки растворимые таблетки белого цвета с элементами мраморности, двояковыпуклой формы.
Вспомогательные вещества: магния стеарат — 3 мг.
30 шт. — упаковки ячейковые контурные (1) — пачки картонные.
Фармакологическое действие
Комбинированное лекарственное средство, регулятор тканевого обмена, обладает антигипоксическим и антиоксидантным свойствами, повышает аппетит, уменьшает токсическое действие этанола.
Стимулируя окислительно-восстановительные реакции, процессы дыхания и синтез АТФ, активирует физиологические функции органов и тканей (стимулирует адаптационные и компенсаторно-защитные возможности организма); повышает умственную и физическую работоспособность; улучшает течение беременности, рост и развитие плода; повышает секрецию желудочного сока, образование соляной кислоты и аппетит; уменьшает токсическое действие алкоголя.
Действие проявляется через 10-20 мин после приема внутрь.
Фармакокинетика
Янтарная и лимонная кислоты полностью метаболизируются до воды и углекислого газа, не кумулируют.
Показания активных веществ препарата
Лимонтар®
Повышение неспецифической реактивности организма в период беременности; профилактика осложнений при гипоксии и гипотрофии плода, невынашивании беременности; алкогольная интоксикация (легкой и средней тяжести); лечение запойных состояний у больных с хроническим алкоголизмом (в составе комплексной терапии); алкогольный абстинентный синдром; астеновегетативный синдром (астения, снижение работоспособности, снижение аппетита); в качестве «пробного завтрака» при исследовании секреторной и кислотообразующей функции желудка.
Режим дозирования
Способ применения и режим дозирования конкретного препарата зависят от его формы выпуска и других факторов. Оптимальный режим дозирования определяет врач. Следует строго соблюдать соответствие используемой лекарственной формы конкретного препарата показаниям к применению и режиму дозирования.
Препарат применяют внутрь, до еды. Перед приемом таблетку измельчают и растворяют в воде с питьевой содой или минеральной воде.
Беременным женщинам назначают по 1 таб./сут в течение 10 дней в I триместре (на сроке беременности 12-14 недель) и во II триместре (срок беременности 24-26 недель). В III триместре назначают за 10-25 дней до родов. Общая доза препарата за период беременности 5-7.5 г.
Для профилактики алкогольной интоксикации — 1 таб. препарата за 20-60 мин до приема алкоголя. При острой алкогольной интоксикации препарат назначают по 1 таб. 2-4 раза/сут с интервалом в 1-2.5 ч. Для купирования запойных состояний при хроническом алкоголизме назначают по 1 таб. 3-4 раза/сут в течение 4-10 дней, как самостоятельно, так и в комплексе с традиционными лекарственными средствами.
В качестве средства для «пробного завтрака» принимают внутрь, натощак, 1 таб. предварительно растворяют в 10-15 мл воды.
Побочное действие
Возможно: гастралгия, гиперсекреция желудочного сока (обычно эти явления проходят самостоятельно через 3-5 мин).
У лиц, склонных к артериальной гипертензии, при систематическом приеме — повышение АД.
Противопоказания к применению
Артериальная гипертензия; ИБС; стенокардия; язвенная болезнь желудка и двенадцатиперстной кишки (в фазе обострения); глаукома; поздний гестоз (тяжелая форма); повышенная чувствительность к компонентам препарата.
Применение при беременности и кормлении грудью
Беременным женщинам препарат назначают по показаниям.
Особые указания
При появлении чувства тяжести в эпигастральной области назначают после еды.
Лекарственное взаимодействие
Ослабляет действие снотворных лекарственных средств, транквилизаторов.
Состав
В химическом составе одной растворимой таблетки Лимонтара содержится: 200 мг янтарной кислоты, 50 мг моногидрата лимонной кислоты, а также 3 мг вспомогательного соединения — стеарата магния.
Форма выпуска
Лимонтар выпускают в таблетированном виде. Мраморно-белые по цвету таблетки, имеющие двояковыпуклую форму, расфасовывают в фигурные блистеры, а затем упаковывают в картонные коробки по 30 штук.
Фармакологическое действие
Это лекарство обладает антиоксидантными свойствами, а также оказывает на организм человека метаболическое и антиалкогольное воздействие.
Фармакодинамика и фармакокинетика
По своей сути Лимонтар – это препарат комплексного воздействия, который выступает в качестве регулятора тканевого обмена. Лекарственное средство способствует усилению окислительно-восстановительных процессов, тем самым оказывая антигипоксическое воздействие и проявляя, таким образом, свои антиоксидантные свойства.
Данное лекарство активирует функции тканей и органов, увеличивает реактивность организма, усиливает выработку желудочного сока, тем самым стимулируя аппетит, купируя токсичное воздействие алкогольных напитков, а также повышая физическую и умственную работоспособность человека. Эффективно помогает избавиться Лимонтар от похмелья, а кроме того препарат используют для профилактики запойных состояний и лечения алкогольной зависимости.
Ко всему прочему, препарат способствует нормальному развитию плода во время беременности. Лекарственное воздействие Лимонтара заметно уже по прошествии 20 минут с момента приема препарата. Активно действующие соединения, входящие в состав лекарства, полностью метаболизируются и в конечном итоге преобразуются в углекислый газ и воду, не скапливаясь в организме.
Показания к применению
Лимонтар принимают:
- при беременности как профилактическое и лекарственное средство;
- при гипотрофии плода, а также при гипоксии;
- при невынашивании беременности;
- для устранения последствий алкогольного опьянения;
- при постинтоксикационных расстройствах, вызванных алкогольными напитками;
- как препарат, входящий в состав комплексной терапии при лечении пациентов с хроническим алкоголизмом, абстинентным синдромом и астено-вегетативными расстройствами;
- в качестве, так называемого, пробного завтрака, который помогает подготовить желудок человека для дальнейшего исследования его кислотообразующей и секреторной функции.
Противопоказания
К абсолютным противопоказаниям использования данного лекарственного средства относятся:
- гиперчувствительность;
- глаукома;
- гестоз;
- стенокардия;
- гипертензия;
- ишемическая болезнь;
- обострение язвы желудка и двенадцатиперстной кишки.
Побочные действия
При приеме препарата могут возникать следующие побочные действия:
- скачки артериального давления у пациентов, страдающих гипертензией;
- болезненные ощущения подложечной области;
- усиление выработки секрета желудочного сока.
Лимонтар, инструкция по применению (Способ и дозировка)
В соответствии с инструкцией по применению Лимонтара препарат принимают внутрь до еды. Таблетки перед непосредственным использованием нужно растолочь и смешав их с пищевой содой (на кончике ножа) растворить в воде (можно использовать минеральную).
Рекомендованы следующие дозировки Лимонтара:
- при беременности – по 1 таблетке в сутки 10 дней в I (на 12-14 неделе), во II (на 24-26 неделе) и в III триместре (за максимум 25 дней до родов);
- при профилактике опьянения – по 1 таблетке до употребления алкогольных напитков (за минимум 20 минут) ;
- для устранения сильного алкогольного опьянения – по 1 таблетке каждые 2,5 часа 4 раза в сутки;
- для предотвращения запойных состояний – по 1 таблетке 4 раза в сутки на протяжении 10 дней;
- как «пробный завтрак» — по 1 таблетки натощак, растворив в 15 мл. простой воды.
Стоит помнить, что общая доза Лимонтара за время беременности не должна превышать 7,5 г.
Передозировка
В настоящее время нет достоверных научных данных.
Взаимодействие
Лимонтар ослабляет эффективность транквилизаторов и снотворного.
Условия продажи
Приобрести препарат в аптеках можно без рецепта.
Условия хранения
Хранить в сухом месте вдали от детей при температуре, которая не превышает 25°С.
Срок годности
3 года.
Особые указания
Если во время лечения пациент начинает ощущать тяжесть в подложечной области, рекомендуется принимать Лимонтар после еды или прекратить использовать препарат.
При беременности и лактации
Беременность является одним из показаний к применению Лимонтара.
Отзывы о Лимонтаре
В открытых источниках практически нет отзывов врачей о Лимонтаре, однако, женщины, принимавшие препарат в большинстве своем положительно отзываются о лекарственном средстве.
Однако есть и негативные отзывы беременных, считающих огромным минусом наличие побочных эффектов, появление которых совсем нежелательно при беременности, когда любое недомогание оказывает сильное влияние как на организм матери, так и будущего ребёнка.
Цена Лимонтара, где купить
Средняя цена Лимонтара в Украине составляет 100 гривен.
- Интернет-аптеки РоссииРоссия
ЗдравСити
-
Лимонтар таблетки растворимые 250мг 30штООО Биотики МНПК
Аптека Диалог
-
Лимонтар таблетки 250мг №30Биотики
Ригла
-
Лимонтар таб. 250мг №30Биотики МНПК
показать еще

