Medical expert of the article
New publications
Preparations
, medical expert
Last reviewed: 10.08.2022
х
All iLive content is medically reviewed or fact checked to ensure as much factual accuracy as possible.
We have strict sourcing guidelines and only link to reputable media sites, academic research institutions and, whenever possible, medically peer reviewed studies. Note that the numbers in parentheses ([1], [2], etc.) are clickable links to these studies.
If you feel that any of our content is inaccurate, out-of-date, or otherwise questionable, please select it and press Ctrl + Enter.
- ATC classification
- Active ingredients
- Indications
- Release form
- Pharmacodynamics
- Pharmacokinetics
- Use during pregnancy
- Contraindications
- Side effects
- Dosing and administration
- Overdose
- Interactions with other drugs
- Storage conditions
- Shelf life
- Pharmacological group
- Pharmachologic effect
- Manufacturer
Paracetamol has a painkiller, as well as antipyretic effect.

Indications Paracetamol
It is used for therapy in such cases:
- various pain syndromes (tooth or headache, myalgia, algomenorrhea, and in addition, arthralgia, neuralgia and migraine);
- state of fever resulting from the development of infectious diseases.
If the need for rapid elimination of inflammation and pain (for example, after a surgical procedure), and in addition, in cases where oral administration of the drug cannot be performed (suspension or tablets), intravenous administration of the substance can be prescribed.
The drug is prescribed for symptomatic treatment, as well as reducing the intensity of pain and inflammation at the time of its use. It does not affect the progression of pathology.

Release form
The release of the drug produced:
- in tablets (in the amount of 6 or 10 pieces inside blister or cell-free plates);
- in the form of a 2.4% syrup (inside 50 ml bottles), as well as 2.4% suspension (in 0.1 l bottles);
- in rectal suppositories with a volume of 0.08, 0.17, and 0.33 g (in the amount of 5 pieces inside a blister pack; in a box — 2 blisters).

Pharmacodynamics
Paracetamol is an analgesic substance of a non-narcotic nature. Its medicinal effects and properties are in the ability to block (mainly inside the CNS) of the elements COX-1, as well as COX-2. At the same time, the substance affects the pain and thermoregulation centers.
The medication does not have anti-inflammatory properties (this effect is extremely small, which allows not to pay attention to it), because its effect on COX is neutralized by enzyme peroxidase inside the inflamed tissues.
Since the drug does not block the binding of Pg inside peripheral tissues, it does not adversely affect the processes of water-electrolyte metabolism inside the body and the mucous membrane of the digestive tract.

Pharmacokinetics
The drug absorption is quite high, its Cmax values are in the range of 5-20 μg / ml. Paracetamol reaches these indicators after 0.5-2 hours. The active element is able to penetrate the BBB.
When breastfeeding, the drug is excreted with the mother’s milk (its concentration does not exceed 1% mark).
The drug is subject to processes of hepatic biotransformation. Metabolism involving microsomal hepatic enzymes leads to the formation of toxic metabolic intermediates (such as N-acetyl-b-benzoquinone imine). These components can lead to damage and necrosis of the liver cells, if there are reduced levels of glutathione in the body. The depletion of stocks of this element is observed when using 10+ g of paracetamol.
Two other metabolic pathways of paracetamol are the process of conjugation with sulfates (often observed in newborns, especially premature), as well as with glucuronides (mostly observed in adults).
Conjugated metabolic products have a weak drug activity (this includes the toxic effect).
Half-life period is within 1-4 hours (for elderly people, this value may be higher). Excretion occurs mainly through the kidneys, in the form of conjugates. Only 3% of used paracetamol is excreted unchanged.

Dosing and administration
Serving sizes for adolescents (from 12 years old, if their weight is more than 40 kg) and adults make up a maximum of 4 g per day (20 tablets with a volume of 0.2 g or 8 tablets with a volume of 0.5 g).
For 1 use should be taken at 0.5 g of the substance (if necessary, may be 1 g). Tablets drugs are allowed to use up to 4 times per day. The duration of therapy is 5-7 days.
Children’s Paracetamol tablets can be consumed from 2 years old. Younger children are required to take 0.5 tablets with a volume of 0.2 g with 4-6-hour intervals. A child older than 6 years old is allowed to take a whole pill, with the frequency indicated above.
Tablets of 325 mg can be taken from 10 years. Children of the age group of 10-12 years of age are prescribed for oral administration 2-3 times per day (the maximum dose should not be exceeded — in this category of patients, it is 1500 mg per day).
Teenagers from 12 years old and adults should take 1-3 tablets with a 4-6 hour interval. You can not take a day more than 4 g of the drug, and the intervals between use should be at least 4 hours.
The use of suppositories.
Suppositories are administered rectally — into the rectum. Before the procedure is necessary to clean the intestines.
The size of the dosage of drugs in suppositories for the child should be calculated, taking into account the age and weight of the patient. 80 mg suppositories are used for infants from 3 months of age; 170 mg suppositories for children 1-6 years old; 330 mg suppositories for children 7-12 years old.
To enter the suppository should be on the first thing, while adhering to a minimum of 4-hour gap between procedures; 3-4 suppositories are administered per day (the number of suppositories depends on the patient’s condition).
Children are often prescribed Paracetamol in suppositories or syrup. When comparing their therapeutic efficacy, it is noted that the syrup has a more rapid, and suppositories have a longer effect.
Because it is safer and more convenient to use the suppository (compared to pills), young children are recommended to prescribe them (for example, for newborns they are considered the optimal dosage form of this medication).
For a child, a toxic dose of drugs is 150+ mg / kg. Thus, a child weighing 20 kg can die from the use of 3 g of substance per day.
Selection of a 1-fold portion is made according to the formula 10-15 mg / kg with 2-3 times daily use (with 4-6-hour intervals). The maximum dosage of medication for a child is 60 mg / kg per day.
Mode of use of the suspension and syrup for children.
Syrup can be prescribed to babies from 3 months old, and the suspension can be applied already from the 1st month of life (because it does not contain sugar).
Sizes of 1-fold portions of syrup for different age groups:
- babies 3-12 months — 0.5-1 teaspoon;
- children 1-6 years old — 1-2 teaspoons;
- children 6-14 years old — 2-4 teaspoons.
The frequency of admission varies from 1-4 times per day (the child must take the drug at least 4-hour intervals).
The dosage of the infant suspension is similar to that used for syrup. The scheme of use of drugs for infants up to 3 months of age is chosen exclusively by the attending doctor.
The sizes of portions of drugs need to be selected, taking into account also the weight of the child. You can prescribe the use of a maximum of 10-15 mg / kg for 1 use and not more than 60 mg / kg per day. Thus, a 3-year-old child with an average weight of 15 kg should be consumed 150-225 mg of medication for 1 dose.
In the absence of a result, if a suspension or syrup is used in the indicated portions, Paracetamol needs to be replaced by some analogue having another active ingredient.
To eliminate the fever, sometimes a combination of paracetamol with analgin is used (if the temperature is between 38.5 ° C and it is difficult to knock it down). The sizes of portions are the following — paracetamol in the dosages indicated above (taking into account age and weight); analgin — 0.3-0.5 mg / kg.
This combination can not be used often, because the use of aspirin leads to irreversible changes in the blood composition.
A “triad” may also be used, which, in addition to paracetamol, includes aspirin with analgin. Paracetamol can also be supplemented with suprastinum with no-spaa, analginum with no-spaa, or suprastinum with analginum.
But shpa (papaverine can also be used instead) helps open spasmodic capillaries, and antihistamines (such as tavegil or suprastin) potentiate the effects of antipyretic drugs.
If you take the drug is required to provide antipyretic effect, it can be used for a maximum of 3 days in a row.
If the drug is used to eliminate pain, the treatment cycle should last a maximum of 5 days. The possibility of its subsequent use is determined by the treating doctor.
It is required to remember that the drug helps to eliminate the symptoms of the disease (such as dental or headaches), but the very cause of their appearance does not cure.

Use Paracetamol during pregnancy
The drug may pass through the placenta, but no negative impact on the development of the fetus has yet been identified.
During the tests, it was determined that the use of Paracetamol during pregnancy (especially in the second half of it) increases the likelihood of asthma, respiratory disorders, wheezing and allergy symptoms in a child.
It should be borne in mind that in the 3rd trimester, the toxic effects of infectious diseases can be just as dangerous as the effects of individual drugs. With hyperthermia, the mother is likely to have hypoxia in the fetus.
When using drugs on the 2nd trimester (more specifically, starting from the 3rd month and approximately until the 18th week), the child may experience abnormalities in the development of internal organs, often manifested after birth. Because of this, the drug in this period is used only sporadically and solely as a last resort.
But at the same time, this particular medicine is considered the safest painkiller for pregnant women.
Taking large portions of the drug during pregnancy can have a negative impact on the kidneys and liver. If a pregnant woman has a fever in connection with SARS or influenza, Paracetamol is required to take 0.5 tablets per use. This therapy should last for a maximum of 7 days.
Only a minimal amount of the drug passes into the mother’s milk during lactation. This allows you to not interrupt breastfeeding in cases where the drug is used for a maximum of 3 days in a row.
During lactation, it is allowed to take a maximum of 3-4 tablets of 0.5 g per day (tablets are taken after the feeding procedure). It is recommended to feed the next time at least 3 hours after drinking the drug.
Contraindications
Main contraindications:
- the presence of intolerance regarding the drug;
- congenital hyperbilirubinemia;
- lack of G6FD enzyme;
- severe hepatic or renal diseases;
- blood diseases;
- alcohol addiction;
- severe anemia or leukopenia.

Side effects Paracetamol
Negative manifestations after taking the drug usually have the form of signs of severe sensitivity (allergies) — pruritus, angioedema, urticaria and rash.
Sometimes the use of the drug leads to the development of disorders of hematopoiesis (thrombocyto-, neutro-, leuco- or pancytopenia, as well as agranulocytosis) and dyspeptic symptoms.
Prolonged use in large portions can provoke a hepatotoxic effect.

Overdose
Manifestations of intoxication that occur on the first day: pallor of the skin, pain in the abdominal zone, nausea, metabolic acidosis, vomiting, anorexia, and the breakdown of glucose metabolism.
After 12–48 o’clock, symptoms of hepatic dysfunction may develop.
In severe overdose, pancreatitis is noted, liver failure (there is progressive encephalopathy on its background), insufficiency of the renal activity in the acute form (accompanied by necrosis of the tubular nature), arrhythmia and comatose state.
In some cases, when poisoning with Paracetamol, death may occur (with very severe intoxication).
For the treatment of disorders, the victim requires the introduction of methionine with acetylcysteine (in the period of 8-9 hours), which are precursors of glutathione binding processes, and with it donators of SH categories.
Subsequent therapy depends on the prescription of the drug and its level inside the blood.

Interactions with other drugs
The drug weakens the effectiveness of uricosuric drugs. Using together with large portions of the drug enhances the effects of anticoagulants (by reducing the production of procoagulants inside the liver).
Drugs that promote the induction of microsome oxidation processes inside the liver, as well as hepatotoxic drugs and ethyl alcohol increase the production of hydroxylated metabolic products with drug activity, which can cause severe poisoning even with minimal overdose.
Drug efficacy decreases with continued use of barbiturates. Ethyl alcohol causes the appearance of pancreatitis in acute form. Drugs that inhibit the oxidation of microsomes inside the liver, reduce the likelihood of hepatotoxic effects.
Prolonged combination with other NSAIDs can provoke necrotic papillitis, analgesic nephropathy, as well as the development of the terminal (dystrophic) stage of kidney failure.
The combination of Paracetamol (in large portions) with salicylates over an extended period of time increases the risk of developing kidney or urinary carcinoma. Diflunisal 50% increases the plasma values of paracetamol, which increases the likelihood of hepatotoxicity.
Myelotoxic substances potentiate the hematotoxic properties of drugs; antispasmodics lead to a delay in its absorption; cholesterol with enterosorbents reduce its bioavailability.

Storage conditions
Paracetamol is required to be kept in a dark and dry place, closed to children. Temperature indicators for syrup — maximum 18 ° C (it can not be frozen); for suppositories, a maximum of 20 ° C.

Shelf life
Paracetamol in suppositories and syrup can be used within 24 months from the moment of release of the medicine. Shelf life of tablets is 36 months.

Analogs
Paracetamol-containing substances such as Strimol, Paracetamol 325, Perfalgan with Paracetamol MC, Cefecon D, Ifimol, Flutabs and Paracetamol Extratab with Panado Daleron, as well as Paracetamol UBF and Efferalgan.
Drugs that have a similar mechanism of action, but a different active ingredient: Antiflu, Coldrex, Antigrippin, Novalgin and Solpadein with Caffetine and Vervex, and in addition, Maxicold, TheraFlu, Panadol Extra and Femizol.

Reviews
Paracetamol is most often mentioned in the context of treating children, since they are more likely to become infected with SARS, and the medicine is most effective in such diseases.
Parents generally leave positive reviews of the drug — it quickly lowers the temperature and reduces the severity of the negative symptoms of fever. At the same time, it is well tolerated by people of different ages — it rarely provokes the development of negative signs typical of NSAIDs.
Doctors are calling to not forget that the medicine removes only the manifestations of the disease, not eliminating it itself, and also remind you that in order to obtain a positive effect it is very important to choose the form of drug release and calculate the required dosage.
Attention!
To simplify the perception of information, this instruction for use of the drug «Paracetamol» translated and presented in a special form on the basis of the official instructions for medical use of the drug. Before use read the annotation that came directly to medicines.
Description provided for informational purposes and is not a guide to self-healing. The need for this drug, the purpose of the treatment regimen, methods and dose of the drug is determined solely by the attending physician. Self-medication is dangerous for your health.
Translation Disclaimer: The original language of this article is Russian. For the convenience of users of the iLive portal who do not speak Russian, this article has been translated into the current language, but has not yet been verified by a native speaker who has the necessary qualifications for this. In this regard, we warn you that the translation of this article may be incorrect, may contain lexical, syntactic and grammatical errors.

Effectively lowers the temperature and eliminates pain syndrome. In addition, it copes well with headache, dental, menstrual pain, symptoms of neuralgia. The main advantage of the drug is low toxicity.
As defined by WHO, it is considered one of the safest and most effective medicines and is widely used to treat children.
Clinical and pharmacological group
Analgetic-antipyretic. Has analgesic, antipyretic and weak anti-inflammatory effect.
Conditions of leave from pharmacies
Released without a doctor’s prescription.
Price list
How much does Paracetamol cost in pharmacies? The average price is at the level of10 rubles.
Form of issue and composition
The following dosage forms of Paracetamol are available:
- Tablets: white with a creamy hue or white, flat-cylindrical, with a risk and facet (10 pcs. in contour mesh or cell-free packages; 2 or 3 packs in a pack of cardboard);
- Suppositories rectal for children: torpedo, from white with a yellowish or creamy hue to white (5 pcs. in packs of cell contour; 2 packs in a pack of cardboard);
- Syrup (100 ml in vials, 1 bottle in a cardboard box);
- Suspension for oral administration (100 ml in bottles of dark glass with a dosage spoon in the kit, 1 set in a pack of cardboard).
1 tablet contains:
- Active substance: paracetamol — 200 or 500 mg;
- Auxiliary components: lactose (milk sugar), stearic acid, potato starch, gelatin.
In 5 ml of syrup contains:
- Active substance: paracetamol — 24 mg;
- Auxiliary components: water, sodium benzoate, aromatic additives, riboflavin, ethyl alcohol, propylene glycol, sodium citric acid trisubstituted, citric acid, sorbitol, sugar.
In 5 ml of the suspension contains:
- Active substance: paracetamol — 120 mg;
- Auxiliary components: purified water, orange or strawberry flavor, sorbitol food (sorbitol), glycerol (glycerin), sucrose (sugar), propylene glycol, methylparahydroxybenzoate (nipagin), xanthan gum (xanthan gum), avicel RC-591 (microcrystalline cellulose, sodium carmellose).
In 1 suppository contains:
- Active substance: paracetamol — 100 mg;
- Auxiliary components: a solid fat base.
Pharmacological effect
Paracetamol belongs to the drugs of the group of analgesics-antipyretics, that is, analgesics and antipyretic agents. In addition to analgesic and antipyretic effect, the drug also has a mild anti-inflammatory effect.
The mechanism of pharmacological action of Paracetamol is related to its ability to slow down the synthesis of prostaglandins and to affect the center of thermoregulation in the hypothalamus. When using the drug, the maximum concentration of the drug in the blood plasma is observed after 10-60 minutes.
Indications for use
What helps? Paracetamol is prescribed for the symptomatic treatment of pain syndrome of mild or moderate severity, of different origin and localization.
However, the most common indication for the beginning of taking this medicine is an increase in body temperature (hyperthermia) against the background of catarrhal and viral diseases, as well as pain (ache) in the bones and muscles of influenza and other acute respiratory viral infections.
Diseases, and pathological conditions in which acetaminophen is recommended:
- neuralgia;
- fever, unspecified genesis;
- sciatica;
- arthrosis;
- toothache;
- headache (incl. migraine);
- arthralgia (joint pain);
- myalgia (muscle pain);
- algodismenorea (painful periods).
Contraindications
Contraindications include:
- individual hypersensitivity (hypersensitivity) to the active substance;
- «Aspirin triad» (combination of intolerance to NSAIDs, bronchial asthma and recurrent polyposis of the nose and paranasal sinuses);
- inflammatory diseases, erosion and ulcers of the gastrointestinal tract;
- gastrointestinal bleeding;
- pronounced functional kidney failure;
- diagnosed hyperkalemia;
- age to 6 years for taking tablets;
- state after coronary artery bypass grafting.
Particular care when taking this drug should be observed in the following diseases and pathological conditions:
- chronic alcoholism and alcoholic liver damage;
- ischemic heart disease and chronic heart failure;
- cerebrovascular disease;
- lesions of peripheral arteries;
- renal and hepatic insufficiency.
At a diabetes it is not recommended to accept Paracetamol in the form of a syrup.
Use in pregnancy and lactation
If you need to use during pregnancy and lactation (breastfeeding), you should carefully weigh the expected benefit of therapy for the mother and the potential risk to the fetus or child.
- Paracetamol penetrates the placental barrier. To date, there has been no adverse effect of paracetamol on the fetus in humans.
- Paracetamol is excreted in breast milk: the content in milk is 0.04-0.23% of the dose taken by the mother.
In experimental studies, embryotoxic, teratogenic and mutagenic effects of paracetamol have not been established.
Dosage and route of administration
The instructions for use indicate that the tablets Paracetamol is prescribed orally.
- Adults and children over 15 years of agesingle dose inside — 500 mg; the maximum single dose is 1000 mg. The maximum daily dose is 4000 mg.
- Ages over 12 years old(with a body weight of more than 40 kg), a single dose of 500 mg, the maximum daily dose of 2000-4000 mg.
- At the age of 9-12 years(body weight up to 40 kg), a dose of 500 mg, a maximum daily dose of 2000 mg.
- Children from 6 to 9 years(with a body weight of 22-30 kg: single dose depends on the weight of the child and is 250 mg, the maximum daily dose of 1000-1500 mg.
The recommended interval between doses of the drug is 6-8 hours (not less than 4 hours).
Duration of treatment is no more than 3 days as an antipyretic agent and not more than 5 days as an anesthetic.
The need to continue treatment with the drug is decided by the doctor.
Side effects
The action of the drug in violation of the instructions, dosage provokes side effects. Overdose can cause:
- dysfunction of the liver or kidneys;
- rashes, redness, «urticaria». Allergy to a drug most often has such external manifestations;
- stomach ache. The stomach reacts so to improper intake or excess of the dose;
- sleepiness, I want to sleep. The cause of the condition is low pressure;
- a sharp drop in the level of glucose, hemoglobin in the blood.
If the dosage is disturbed or improperly taken, immediately call an ambulance.
Overdose
With prolonged use of tablets in large doses, the patient quickly develops symptoms of overdose, which clinically manifested in the form of strengthening of the above-described side effects and the development of hepatic insufficiency.
If you randomly ingest a large number of tablets, the patient should rinse the stomach as soon as possible and deliver it to the hospital. If necessary, symptomatic treatment is performed. Paracetamol antidote is H-acetylestein, it is administered orally or intravenously.
special instructions
With prolonged use of paracetamol, monitoring of the peripheral blood pattern and the functional state of the liver is necessary.
Caution is used in patients with impaired liver and kidney function, with benign hyperbilirubinemia, as well as in elderly patients.
It is used for the treatment of premenstrual tension syndrome in combination with pamabrom (diuretic, xanthine derivative) and mepirin (histamine H1 receptor blocker).
Drug Interactions
With simultaneous application:
- activated carbon decreases the bioavailability of paracetamol.
- with uricosuric means their effectiveness decreases.
- with diazepam, a decrease in the excretion of diazepam is possible.
- with carbamazepine, phenytoin, phenobarbital, primidone decreases the effectiveness of paracetamol, which is due to increase of its metabolism (processes of glucuronization and oxidation) and excretion from the body. The cases of hepatotoxicity with simultaneous application of paracetamol and phenobarbital are described.
- for a period of less than 1 hour after paracetamol administration, a decrease in absorption of paracetamol is possible.
- lamotrigine moderately increases the excretion of lamotrigine from the body.
- with anticoagulants, a slight or moderate increase in prothrombin time is possible.
- with anticholinergic agents, a reduction in paracetamol absorption is possible.
- with oral contraceptives, the excretion of paracetamol from the body is accelerated and its analgesic effect may decrease.
- with metoclopramide, it is possible to increase the absorption of paracetamol and increase its concentration in the blood plasma.
- with probenecid, a decrease in paracetamol clearance is possible; with rifampicin, sulfinpyrazone — it is possible to increase the clearance of paracetamol due to an increase in its metabolism in the liver.
- with inducers of microsomal liver enzymes, agents that have a hepatotoxic effect, there is a risk of intensifying the hepatotoxic effect of paracetamol.
- With ethinyl estradiol, paracetamol is absorbed from the intestine.
Besides:
- Cases of toxic effects of paracetamol when used with isoniazid are described.
- There are reports of the possibility of enhancing the myelodepressant effect of zidovudine when used concomitantly with paracetamol. A case of severe toxic liver damage is described.
Reviews
We picked up some reviews of people who used the drug Paracetamol:
- Igor. Paracetamol is always with me, I often am on the road, work is connected with traveling and this drug is constantly available in the car kit. He saved me many times. I take it with headache and toothache, with a cold. Good temperature, quickly improves well-being and unlike aspirin does not have a harmful effect on the stomach. It is quite inexpensive and is sold in any pharmacy.
- Margarita. On the problem skin I know firsthand. I treat acne with a bolt, which I do on the basis of paracetamol and boric acid. In combination, these drugs perfectly relieve inflammation, remove redness and dry the rash. I heard that very good tablets eliminate toothache and stomach cramps with menstruation.
- Sasha. Tablet Paracetamol and do not need additional medicine for the temperature, and all these powders, which he enters, just bullshit, diluted with any additional chemistry to sell more expensive. All these Nurofens, Teraflu and others. Drink pure Paracetamol and there will be happiness and health.
Analogues
Paracetamol has many analogues containing the same active substance. They are produced by many pharmaceutical companies under different brands. Here we give only some analogues of Paracetamol:
- Acetophene
- Aminadol
- Aminofen
- Apamid
- Apanol
- Biocetamol
- Valadol
- Valorine
- Deminofen
- Dolamine
- Metamol
- Mialgin
- Paramol
- Panadol Junior
- Pirinazine
- TempraMol
- Febridol
- Hemcetaphene
- Celiphane
- Efferalgan
Before using analogues, consult your doctor.
Which is better: Paracetamol or Ibuprofen?
Ibuprofen (Nurofen) has a broader spectrum of action and more favorably, in comparison with Paracetamol, affects the temperature curve. The effect from its application comes faster (already in 15-25 minutes) and lasts longer (up to 8 hours), in addition, the drug is considered less harmful and less likely to provoke allergic reactions. Ibuprofen better than its counterpart removes the critically high temperature. Repeatedly (to control hyperthermia) it is used much less often than Paracetamol.
The power of antipyretic action is comparable, however, ibuprofen, in addition to anesthetic and antipyretic action, also effectively removes inflammation in peripheral tissues. This is due to the fact that paracetamol acts primarily in the central nervous system, and ibuprofen — suppresses the synthesis of Pg not so much in the central nervous system as in inflamed peripheral tissues. That is, with expressed peripheral inflammation, the choice should be made in favor of Nurofen and other drugs based on ibuprofen.
Answering the question «What to choose, Paracetamol or Nurofen?», Doctors recommend starting treatment of young children with monotherapy with Ibuprofen. If you need to urgently reduce the temperature, you can use any of the drugs. Follow-up treatment should be agreed with the doctor. It should be noted that suppositories with ibuprofen are contraindicated in children weighing up to 6 kg, and suspension for children up to 3 months.
Storage conditions and shelf life
The drug in any dosage forms is released without the appointment of a doctor. Shelf life of Paracetamol is:
- Suppositories — 2 years at a temperature of up to 15 ° C;
- Tablets — 3 years at a temperature of up to 25 ° C;
- Syrup, solution for oral administration and suspension — 2 years at a temperature of up to 25 ° C.
Keep away from children!
Paracetamol pharmacokinetic parameters
| Oral bioavailability (F) | 70%-90% |
| Rectal bioavailability (F) | 30%-70% |
| Clearance (CL) | 20 L/h |
| Volume of Distribution (Vd) | 65 L |
| Half-life (t1/2) | 2.5 h |
Description
Paracetamol (also called acetaminophen) is a widely used analgesic and antipyretic agent.
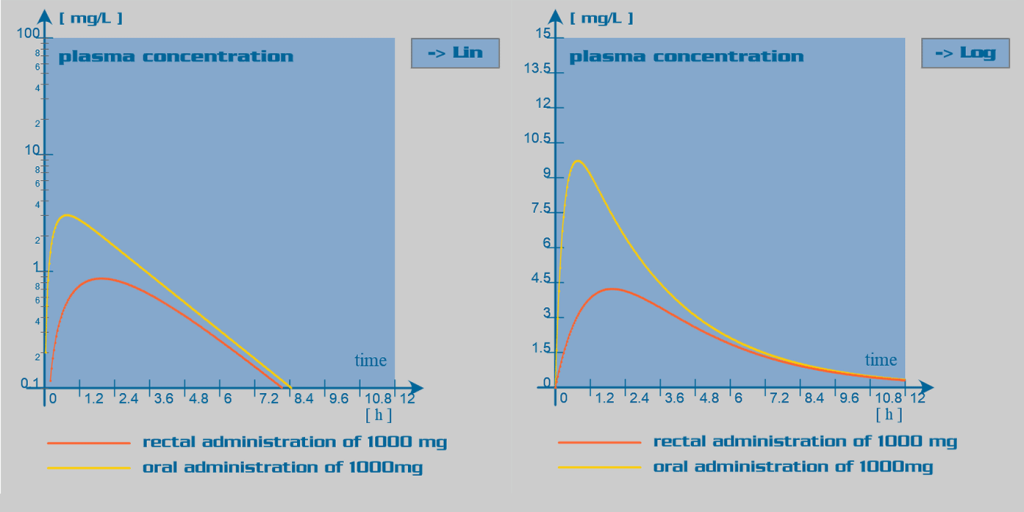
Paracetamol is well absorbed in the gastrointestinal tract. Oral bioavailability is dose dependant: with larger doses, the hepatic first pass effect
is reduced due to overwhelming of the liver enzymatic capacity; and
therefore, bioavailability is increased. Rectal administration of
paracetamol is also feasible. In this case, bioavailability is
inconsistent and in overall reduced, due to incomplete dissolution of
the suppository in the rectum. The absorption rate through this route of administration is elongated.
Paracetamol is distributed
throughout the body fluids in a homogeneous way. The analgesic activity
is attributable to the small fraction that penetrates into the brain.
Paracetamol given at therapeutic doses binds to plasma proteins at less than 20%. In case of intoxication, this proportion may increase to up to 50%.
Paracetamol is essentially metabolized
in the liver by conjugation with glucuronic acid (55%) and sulfuric
acid (35%). Hepatotoxic metabolites are produced in small amounts by the
cytochrome P450 (isoenzyme CYP2E1). In the therapeutic plasma
concentration range, this metabolite is detoxified by conjugation with
glutathione. In case of intoxication the amount of this toxic metabolite
increases and outweighs the amount of available glutathion, which can
lead to hepatic failure and renal tubular necrosis.
Metabolites are excreted
through the kidneys in the urine. Only 2-5% of the dose is excreted in
an unchanged form in the urine. As a consequence of its short
elimination half-life (1-3h), 24 hours after the ingestion of a single dose of paracetamol, 98% of the dose is eliminated.
Clinical implications
Since paracetamol is mostly eliminated by hepatic metabolism, patients with severe hepatic failure have a considerably longer elimination half-life, and therefore, paracetamol must be used cautiously in such patients.
Intoxication are not infrequent because paracetamol
is one of the most widely used over-the-counter drugs. Paracetamol
intoxication has important clinical implications as it can induce
life-threatening hepatic and renal failure. This failure is probably due
to the accumulation of toxic metabolites, caused by an increase in
their production and a decrease in their detoxification due to depletion
of glutathione stores. The treatment of intoxication is:
- gastric emptying and administration of active charcoal
- administration
of N-acetylcysteine, which acts by replenishing the hepatic stores of
glutathione (preferably by the oral route, otherwise intravenously) - heamodialysis may be useful if started within 12 hours after ingestion of huge doses.
Top 20 medicines with the same components:
Top 20 medicines with the same treatments:
Name of the medicinal product
The information provided in Name of the medicinal product of Парацетамол
is based on data of another medicine with exactly the same composition as the Парацетамол.
. Be careful and be sure to specify the information on the section Name of the medicinal product in the instructions to the drug Парацетамол directly from the package or from the pharmacist at the pharmacy.
more…
Парацетамол
Qualitative and quantitative composition
The information provided in Qualitative and quantitative composition of Парацетамол
is based on data of another medicine with exactly the same composition as the Парацетамол.
. Be careful and be sure to specify the information on the section Qualitative and quantitative composition in the instructions to the drug Парацетамол directly from the package or from the pharmacist at the pharmacy.
more…
Acetaminophen
Pharmaceutical form
The information provided in Pharmaceutical form of Парацетамол
is based on data of another medicine with exactly the same composition as the Парацетамол.
. Be careful and be sure to specify the information on the section Pharmaceutical form in the instructions to the drug Парацетамол directly from the package or from the pharmacist at the pharmacy.
more…
Tablet; Oral syrup; Vaginal / rectal suppositories
Therapeutic indications
The information provided in Therapeutic indications of Парацетамол
is based on data of another medicine with exactly the same composition as the Парацетамол.
. Be careful and be sure to specify the information on the section Therapeutic indications in the instructions to the drug Парацетамол directly from the package or from the pharmacist at the pharmacy.
more…
Pills; Rectal suppositories; Rectal suppositories for children; Solution for infusion; Substance; Substance-powder; Syrup; Tablets, effervescent
Bolus; Coated tablet; Effervescent tablet; Film-coated tablet; Tablets, soluble
Suspension for ingestion for children
For the treatment of mild to moderate pain including headache, migraine, neuralgia, toothache, sore throat, period pains, aches and pains, symptomatic relief of rheumatic aches and pains and of influenza, feverishness and feverish colds.
Парацетамол ActiFast is a mild analgesic and antipyretic, and is recommended for the treatment of most painful and febrile conditions, for example, headache including migraine and tension headaches, toothache, backache, rheumatic and muscle pains, dysmenorrhoea, sore throat, and for relieving the fever, aches and pains of colds and flu.
Парацетамол Infant Sugar Free Colour Free 120 mg/5 ml Oral Suspension is indicated for the treatment of mild to moderate pain and as an antipyretic. It can be used in many conditions including headache, toothache, earache, teething, sore throat, colds and influenza, aches and pains and post-immunisation fever.
Dosage (Posology) and method of administration
The information provided in Dosage (Posology) and method of administration of Парацетамол
is based on data of another medicine with exactly the same composition as the Парацетамол.
. Be careful and be sure to specify the information on the section Dosage (Posology) and method of administration in the instructions to the drug Парацетамол directly from the package or from the pharmacist at the pharmacy.
more…
Pills; Rectal suppositories; Rectal suppositories for children; Solution for infusion; Substance; Substance-powder; Syrup; Tablets, effervescent
Bolus; Coated tablet; Effervescent tablet; Film-coated tablet; Tablets, soluble
Suspension for ingestion for children
Adults, the elderly and young persons 16 years and over:
2 tablets every 4 hours to a maximum of 8 tablets in 24 hours.
Children 6 — 9 years:
½ tablet every 4 hours to a maximum of 4 doses in 24 hours.
Children 10 — 11 years:
1 tablet every 4 hours to a maximum of 4 doses in 24 hours
Adolescents 12 — 15 years:
1 to 1 ½ tablets every 4 hours to a maximum of 4 doses in 24 hours
Do not give to children aged under 6 years of age.
For oral administration.
Adults, including the elderly and children 16 years and over:
Two tablets to be taken with half a tumbler of water (100 ml).
To ensure fast onset of pain relief no less than two tablets must be taken with 100 ml of water. For maximum speed of action this should be on an empty stomach.
Two tablets up to four times daily as required. The dose should not be repeated more frequently than every four hours nor should more than four doses be taken in any 24 hour period.
Children aged 12-15 years:
One tablet to be taken with half a tumbler of water (100ml), up to four times daily as required. The dose should not be repeated more frequently than every four hours nor should more than 4 doses be given in any 24 hour period.
Children under 12 years of age:
Парацетамол ActiFast is not recommended for children under 12 years of age.
For the relief of fever after vaccinations at 2, 3 and 4 months
2.5ml. This dose may be given up to 4 times a day starting at the time of vaccination. Do not give more than 4 doses in any 24 hour period. Leave at least 4 hours between doses. If your baby still needs this medicine two days after receiving the vaccine talk to your doctor or pharmacist.
|
Age : 2 — 3 months |
Dose |
|
Pain and other causes of fever — if your baby weighs over 4 kg and was born after 37 weeks |
2.5 ml If necessary, after 4-6 hours, give a second 2.5 ml dose |
|
— Do not give to babies less than 2 months of age. — Leave at least 4 hours between doses. — Do not give more than 2 doses. This is to ensure that fever that may be due to a serious infection is quickly diagnosed. If your child is still feverish after two doses, talk to your doctor or pharmacist. |
Children aged 3 months — 6 years:
|
Child’s Age |
How Much |
How often (in 24 hours) |
|
3 — 6 months |
2.5 ml |
4 times |
|
6 — 24 months |
5 ml |
4 times |
|
2 — 4 years |
7.5 ml (5 ml + 2.5 ml) |
4 times |
|
4 — 6 years |
10 ml (5 ml + 5 ml) |
4 times |
|
— Do not give more than 4 doses in any 24 hour period — Leave at least 4 hours between doses — Do not give this medicine to your child for more than 3 days without speaking to your doctor or pharmacist |
It is important to shake the bottle for at least 10 seconds before use.
The Elderly:
In the elderly, the rate and extent of paracetamol absorption is normal but plasma half-life is longer and paracetamol clearance is lower than in young adults.
Contraindications
The information provided in Contraindications of Парацетамол
is based on data of another medicine with exactly the same composition as the Парацетамол.
. Be careful and be sure to specify the information on the section Contraindications in the instructions to the drug Парацетамол directly from the package or from the pharmacist at the pharmacy.
more…
Pills; Rectal suppositories; Rectal suppositories for children; Solution for infusion; Substance; Substance-powder; Syrup; Tablets, effervescent
Bolus; Coated tablet; Effervescent tablet; Film-coated tablet; Tablets, soluble
Suspension for ingestion for children
Hypersensitivity to Парацетамол or any of the constituents.
Hypersensitivity to paracetamol or any of the other constituents.
Special warnings and precautions for use
The information provided in Special warnings and precautions for use of Парацетамол
is based on data of another medicine with exactly the same composition as the Парацетамол.
. Be careful and be sure to specify the information on the section Special warnings and precautions for use in the instructions to the drug Парацетамол directly from the package or from the pharmacist at the pharmacy.
more…
Pills; Rectal suppositories; Rectal suppositories for children; Solution for infusion; Substance; Substance-powder; Syrup; Tablets, effervescent
Bolus; Coated tablet; Effervescent tablet; Film-coated tablet; Tablets, soluble
Suspension for ingestion for children
Care is advised in the administration of Парацетамол to patients with severe renal or severe hepatic impairment. The hazards of overdose are greater in those with non-cirrhotic alcoholic liver disease.
Do not take more medicine than the label tells you to. If you do not get better, talk to your doctor.
Contains Парацетамол.
Do not take anything else containing Парацетамол while taking this medicine.
Talk to your doctor at once if you take too much of this medicine, even if you feel well. This is because too much Парацетамол can cause delayed, serious liver damage.
Patients should be advised that Парацетамол may cause severe skin reactions. If a skin reaction such as skin reddening, blisters, or rash occurs, they should stop use and seek medical assistance right away.
Care is advised in the administration of paracetamol to patients with renal or hepatic impairment. The hazard of overdose is greater in those with non-cirrhotic alcoholic liver disease.
Do not exceed the stated dose.
Patients should be advised not to take other paracetamol-containing products concurrently.
Each Парацетамол ActiFast tablet contains 173 mg of sodium and should not be taken by patients on a low sodium diet.
Patients should be advised to consult their doctor if their headaches become persistent.
If symptoms persist consult your doctor.
Keep out of the reach and sight of children.
Pack Label:
Immediate medical advice should be sought in the event of an overdose, even if you feel well.
Do not take with any other paracetamol-containing products.
Patient Information Leaflet:
Immediate medical advice should be sought in the event of an overdose, even if you feel well, because of the risk of delayed, serious liver damage.
Парацетамол Infant Sugar Free Colour Free 120 mg/5 ml Oral Suspension should be used with caution in severe renal impairment or severe hepatic impairment. The hazards of overdose are greater in those with non-cirrhotic alcoholic liver disease.
Concomitant use of other paracetamol-containing products should be avoided.
Due to the presence of maltitol liquid (E965) and sorbitol liquid (E420), patients with rare hereditary problems of fructose intolerance should not take this medicine.
Ethyl (E214), Propyl (E216) and Methyl (E218) parahydroxybenzoate may cause allergic reactions (possibly delayed).
Patients should be informed about the signs of serious skin reactions, and use of the drug should be discontinued at the first appearance of skin rash or any other sign of hypersensitivity.
The label contains the following statements:
Contains paracetamol.
Do not give anything else containing paracetamol while giving this medicine.
Do not give more medicine than the label tells you to. If your child does not get better, talk to your doctor.
For oral use only.
Always use the syringe supplied with the pack.
Do not give to babies less than 2 months of age.
For infants 2-3 months no more than 2 doses should be given.
Do not give more than 4 doses in any 24 hour period.
Leave at least 4 hours between doses.
Do not give this medicine to your child for more than 3 days without speaking to your doctor or pharmacist.
As with all medicines, if your child is currently taking any other medicine consult your doctor or pharmacist before using this product.
Keep out of the sight and reach of children.
Do not store above 25°C. Keep bottle in the outer carton.
It is important to shake the bottle for at least 10 seconds before use.
Talk to a doctor at once if your child takes too much of this medicine, even if they seem well.
The leaflet contains the following statements:
Talk to a doctor at once if your child takes too much of this medicine, even if they seem well. This is because too much paracetamol can cause delayed, serious liver damage.
Talk to your doctor: If your child has an inherited intolerance to fructose or been diagnosed with an intolerance to some other sugars.
The sorbitol liquid (E420) and maltitol liquid (E965) content of this product means that this product is unsuitable for people with inherited intolerance to fructose.
Very rare cases of serious skin reactions have been reported. Symptoms may include:
— Skin reddening
— Blisters
— Rash
If skin reactions occur or existing skin symptoms worsen, stop use and seek medical help right away.
Effects on ability to drive and use machines
The information provided in Effects on ability to drive and use machines of Парацетамол
is based on data of another medicine with exactly the same composition as the Парацетамол.
. Be careful and be sure to specify the information on the section Effects on ability to drive and use machines in the instructions to the drug Парацетамол directly from the package or from the pharmacist at the pharmacy.
more…
None.
Undesirable effects
The information provided in Undesirable effects of Парацетамол
is based on data of another medicine with exactly the same composition as the Парацетамол.
. Be careful and be sure to specify the information on the section Undesirable effects in the instructions to the drug Парацетамол directly from the package or from the pharmacist at the pharmacy.
more…
Pills; Rectal suppositories; Rectal suppositories for children; Solution for infusion; Substance; Substance-powder; Syrup; Tablets, effervescent
Bolus; Coated tablet; Effervescent tablet; Film-coated tablet; Tablets, soluble
Suspension for ingestion for children
Adverse effects of Парацетамол are rare. Very rare cases of serious skin reactions have been reported. There have been reports of blood dyscrasias including thrombocytopenia purpura, methaemoglobenaemia and agranulocytosis, but these were not necessarily causality related to Парацетамол.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at www.mhra.gov.uk/yellowcard.
Adverse events of paracetamol from historical clinical trial data are both infrequent and from small patient exposure. Accordingly, events reported from extensive post-marketing experience at therapeutic/labelled dose and considered attributable are tabulated below by system class. Due to limited clinical trial data, the frequency of these adverse events is not known (cannot be estimated from available data), but post-marketing experience indicates that adverse reactions to paracetamol are rare and serious reactions are very rare.
Post marketing data
|
Body System |
Undesirable effect |
|
Blood and lymphatic system disorders |
Thrombocytopenia Agranulocytosis |
|
Immune system disorders |
Anaphylaxis Cutaneous hypersensitivity reactions including skin rashes, angiodema and Stevens Johnson syndrome/toxic epidermal necrolysis |
|
Respiratory, thoracic and mediastinal disorders |
Bronchospasm* |
|
Hepatobiliary disorders |
Hepatic dysfunction |
* There have been cases of bronchospasm with paracetamol, but these are more likely in asthmatics sensitive to aspirin or other NSAIDs.
Adverse effects of paracetamol are rare but hypersensitivity/anaphylactic reactions including skin rash may occur. Very rare cases of serious skin reactions have been reported. There have been reports of blood dyscrasias including thrombocytopenia and agranulocytosis but these were not necessarily causally related to paracetamol.
Most reports of adverse reactions to paracetamol relate to overdose with the drug.
Chronic hepatic necrosis has been reported in a patient who took daily therapeutic doses of paracetamol for about a year and liver damage has been reported after daily ingestion of excessive amounts for shorter periods. A review of a group of patients with chronic active hepatitis failed to reveal differences in the abnormalities of liver function in those who were long-term users of paracetamol nor was the control of their disease improved after paracetamol withdrawal.
Nephrotoxicity following therapeutic doses of paracetamol is uncommon, but papillary necrosis has been reported after prolonged administration.
Low level transaminase elevations may occur in some patients taking therapeutic doses of paracetamol; these are not accompanied with liver failure and usually resolve with continued therapy or discontinuation of paracetamol.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard
Overdose
The information provided in Overdose of Парацетамол
is based on data of another medicine with exactly the same composition as the Парацетамол.
. Be careful and be sure to specify the information on the section Overdose in the instructions to the drug Парацетамол directly from the package or from the pharmacist at the pharmacy.
more…
Pills; Rectal suppositories; Rectal suppositories for children; Solution for infusion; Substance; Substance-powder; Syrup; Tablets, effervescent
Bolus; Coated tablet; Effervescent tablet; Film-coated tablet; Tablets, soluble
Suspension for ingestion for children
Liver damage is possible in adults who have taken 10g or more of Парацетамол. Ingestion of 5g or more of Парацетамол may lead to liver damage if the patient has risk factors (see below).
Risk Factors
If the patient
a) Is on long term treatment with carbamazepine, phenobarbitone, phenytoin, primidone, rifampicin, St John’s Wort or other drugs that induce liver enzymes.
Or
b) Regularly consumes ethanol in excess of recommended amounts.
Or
c) Is likely to be glutathione deplete e.g. eating disorders, cystic fibrosis, HIV infection, starvation, cachexia.
Symptoms
Symptoms of Парацетамол overdosage in the first 24 hours are pallor, nausea, vomiting, anorexia and abdominal pain. Liver damage may become apparent 12 to 48 hours after ingestion. Abnormalities of glucose metabolism and metabolic acidosis may occur. In severe poisoning, hepatic failure may progress to encephalopathy, haemorrhage, hypoglycaemia, cerebral oedema, and death. Acute renal failure with acute tubular necrosis, strongly suggested by loin pain, haematuria and proteinuria, may develop even in the absence of severe liver damage. Cardiac arrhythmias and pancreatitis have been reported.
Management
Immediate treatment is essential in the management of Парацетамол overdose. Despite a lack of significant early symptoms, patients should be referred to hospital urgently for immediate medical attention. Symptoms may be limited to nausea or vomiting and may not reflect the severity of overdose or the risk of organ damage. Management should be in accordance with established treatment guidelines, see BNF overdose section.
Treatment with activated charcoal should be considered if the overdose has been taken within 1 hour. Plasma Парацетамол concentration should be measured at 4 hours or later after ingestion (earlier concentrations are unreliable).
Treatment with N-acetylcysteine may be used up to 24 hours after ingestion of Парацетамол however, the maximum protective effect is obtained up to 8 hours post ingestion.
If required the patient should be given intravenous-N-acetylcysteine, in line with the established dosage schedule. If vomiting is not a problem, oral methionine may be a suitable alternative for remote areas, outside hospital.
Management of patients who present with serious hepatic dysfunction beyond 24 hours from ingestion should be discussed with the NPIS or a liver unit.
Liver damage is possible in adults who have taken 10g or more of paracetamol. Ingestion of 5g or more of paracetamol may lead to liver damage if the patient has risk factors (see below).
Risk factors
If the patient
a, Is on long term treatment with carbamazepine, phenobarbitone, phenytoin, primidone, rifampicin, St John’s Wort or other drugs that induce liver enzymes.
Or
b, Regularly consumes ethanol in excess of recommended amounts.
Or
c, Is likely to be glutathione deplete e.g. eating disorders, cystic fibrosis, HIV infection, starvation, cachexia.
Symptoms
Symptoms of paracetamol overdosage in the first 24 hours are pallor, nausea, vomiting, anorexia and abdominal pain. Liver damage may become apparent 12 to 48 hours after ingestion. Abnormalities of glucose metabolism and metabolic acidosis may occur. In severe poisoning, hepatic failure may progress to encephalopathy, haemorrhage, hypoglycaemia, cerebral oedema, and death. Acute renal failure with acute tubular necrosis, strongly suggested by loin pain, haematuria and proteinuria, may develop even in the absence of severe liver damage. Cardiac arrhythmias and pancreatitis have been reported.
Management
Immediate treatment is essential in the management of paracetamol overdose. Despite a lack of significant early symptoms, patients should be referred to hospital urgently for immediate medical attention. Symptoms may be limited to nausea or vomiting and may not reflect the severity of overdose or the risk of organ damage. Management should be in accordance with established treatment guidelines, see BNF overdose section.
Treatment with activated charcoal should be considered if the overdose has been taken within 1 hour. Plasma paracetamol concentration should be measured at 4 hours or later after ingestion (earlier concentrations are unreliable). Treatment with N-acetylcysteine may be used up to 24 hours after ingestion of paracetamol, however, the maximum protective effect is obtained up to 8 hours post-ingestion. The effectiveness of the antidote declines sharply after this time. If required the patient should be given intravenous N-acetylcysteine, in line with the established dosage schedule. If vomiting is not a problem, oral methionine may be a suitable alternative for remote areas, outside hospital. Management of patients who present with serious hepatic dysfunction beyond 24h from ingestion should be discussed with the NPIS or a liver unit.
High doses of sodium bicarbonate may be expected to induce gastrointestinal symptoms including belching and nausea. In addition, high doses of sodium bicarbonate may cause hypernatraemia; electrolytes should be monitored and patients managed accordingly.
Liver damage is possible in adults who have taken 10g or more of paracetamol. Ingestion of 5g or more of paracetamol may lead to liver damage if the patient has risk factors (see below)
Risk Factors:
If the patient
a) Is on long term treatment with carbamazepine, phenobarbitone, phenytoin, primidone, rifampicin, St John’s Wort or other drugs that induce liver enzymes
OR
b) Regularly consumes ethanol in excess of recommended amounts
OR
c) Is likely to be glutathione deplete e.g, eating disorders, cystic fibrosis, HIV infection, starvation, cachexia
Symptoms
Symptoms of paracetamol overdosage in the first 24 hours are pallor, nausea, hyperhidrosis, malaise, vomiting, anorexia, and abdominal pain. Liver damage may become apparent 12 to 48 hours after ingestion. This may include hepatomegaly, liver tenderness, jaundice, acute hepatic failure and hepatic necrosis. Abnormalities of glucose metabolism and metabolic acidosis may occur. Blood bilirubin, hepatic enzymes, INR, prothrombin time, blood phosphate and blood lactate may be increased. In severe poisoning, hepatic failure may progress to encephalopathy, haemorrhage, hypoglycaemia, cerebral oedema and death. Acute renal failure with acute tubular necrosis, strongly suggested by loin pain, haematuria and proteinuria, may develop even in the absence of severe liver damage. Cardiac arrhythmias and pancreatitis have been reported.
Management
Immediate treatment is essential in the management of paracetamol overdose. Despite a lack of significant early symptoms, patients should be referred to hospital urgently for immediate medical attention. Symptoms may be limited to nausea or vomiting and may not reflect the severity of the overdose or the risk of organ damage. Management should be in accordance with established treatment guidelines, see BNF overdose section.
Treatment with activated charcoal should be considered if the overdose has been taken within 1 hour. Plasma paracetamol concentrations should be measured at 4 hours or later after ingestion (earlier concentrations are unreliable). Treatment with N-acetylcysteine may be used up to 24 hours after ingestion of paracetamol, however the maximum protective effect is obtained up to 8 hours post-ingestion. The effectiveness of the antidote declines sharply after this time. If required the patient should be given intravenous N-acetylcysteine, in line with the established dosage schedule. If vomiting is not a problem oral methionine may be a suitable alternative for remote areas, outside hospital. Management of patient who present with serious hepatic dysfunction beyond 24h from ingestion should be discussed with the NPIS or a liver unit.
Pharmacodynamic properties
The information provided in Pharmacodynamic properties of Парацетамол
is based on data of another medicine with exactly the same composition as the Парацетамол.
. Be careful and be sure to specify the information on the section Pharmacodynamic properties in the instructions to the drug Парацетамол directly from the package or from the pharmacist at the pharmacy.
more…
Pills; Rectal suppositories; Rectal suppositories for children; Solution for infusion; Substance; Substance-powder; Syrup; Tablets, effervescent
Bolus; Coated tablet; Effervescent tablet; Film-coated tablet; Tablets, soluble
Suspension for ingestion for children
Mechanisms of Action/Effect
Analgesic — the mechanism of analgesic action has not been fully determined. Парацетамол may act predominantly by inhibiting prostaglandin synthesis in the central nervous system (CNS) and to a lesser extent, through a peripheral action by blocking pain-impulse generation.
The peripheral action may also be due to inhibition of prostaglandin synthesis or to inhibition of the synthesis or actions of other substances that sensitise pain receptors to mechanical or chemical stimulation.
Antipyretic — Парацетамол probably produces antipyresis by acting centrally on the hypothalamic heat-regulation centre to produce peripheral vasodilation resulting in increased blood flow through the skin, sweating and heat loss. The central action probably involves inhibition of prostaglandin synthesis in the hypothalamus.
ATC Code N02B E01
Paracetamol has analgesic and antipyretic actions. The mechanism of action is based on the inhibition of prostaglandin biosynthesis.
Paracetamol is poorly absorbed in the stomach but well absorbed in the small intestine due to the greater surface area and hence adsorptive capacity.
Sodium bicarbonate is an excipient in the formulation which has a role in increasing the rates of gastric emptying and of paracetamol dissolution and hence the speed of absorption of paracetamol to provide faster onset of relief.
The amount of sodium bicarbonate contained in 2 tablets of Парацетамол ActiFast are required per dose to have such effects. Sodium bicarbonate influences the rate of gastric emptying in a concentration dependant manner with the maximal effect achieved at near isotonic concentrations (150 mmol/litre)(i.e. 150 millimolar) — equivalent to 2 Парацетамол ActiFast tablets in 100 ml water.
Hypertonic solutions (500-1,000 mmol/litre)(i.e. 500 to 1,000 millimolar — equivalent to the amount of sodium bicarbonate in 6-12 Парацетамол ActiFast tablets given with 100 ml water) appear to inhibit gastric emptying. The therapeutic application of enhanced gastric emptying has previously been demonstrated with significantly faster rate of absorption of paracetamol and significantly faster onset of pain relief from soluble tablets containing sodium bicarbonate compared to conventional tablets. Парацетамол ActiFast has been formulated with 630 mg sodium bicarbonate per tablet that results in near isotonicity at a 2-tablet dose in gastric fluid.
The role of the dissolution rate of Парацетамол ActiFast Tablets in vivo at gastric pH is unknown. Therefore the role of tablet dissolution in the speed of action of Парацетамол ActiFast Tablets is unclear.
It is likely that no single mode of action is responsible for the pharmacokinetic profile observed with Парацетамол ActiFast. The relative contributions of the different factors will vary depending on the circumstances under which the product is taken.
Pharmacotherapeutic group: Other Analgesics and Antipyretics (Anilides)
ATC Code: N02 BE01
Paracetamol has analgesic and antipyretic effects similar to those of aspirin and is useful in the treatment of mild to moderate pain. It has only weak anti-inflammatory effects.
Pharmacokinetic properties
The information provided in Pharmacokinetic properties of Парацетамол
is based on data of another medicine with exactly the same composition as the Парацетамол.
. Be careful and be sure to specify the information on the section Pharmacokinetic properties in the instructions to the drug Парацетамол directly from the package or from the pharmacist at the pharmacy.
more…
Pills; Rectal suppositories; Rectal suppositories for children; Solution for infusion; Substance; Substance-powder; Syrup; Tablets, effervescent
Bolus; Coated tablet; Effervescent tablet; Film-coated tablet; Tablets, soluble
Suspension for ingestion for children
Absorption and Fate
Парацетамол is readily absorbed from the gastro-intestinal tract with peak plasma concentrations occurring about 30 minutes to 2 hours after ingestion. It is metabolised in the liver and excreted in the urine mainly as the glucuronide and sulfate conjugates. Less than 5% is excreted as unchanged Парацетамол. The elimination half-life varies from about 1 to 4 hours. Plasma-protein binding is negligible at usual therapeutic concentrations but increases with increasing concentrations.
A minor hydroxylated metabolite which is usually produced in very small amounts by mixed-function oxidases in the liver and which is usually detoxified by conjugation with liver glutathione may accumulate following Парацетамол overdosage and cause liver damage.
Paracetamol is rapidly and almost completely absorbed from the gastrointestinal tract. It is metabolised in the liver and excreted in the urine as the glucuronide and sulphate conjugates, — less than 5% is excreted unchanged in the urine as unmodified paracetamol. Binding to plasma proteins is minimal.
The mean elimination half-life of paracetamol following administration of Парацетамол ActiFast is 2 to 3 hours and is similar to that achieved following administration of standard paracetamol tablets in fasted and fed states.
Following administration of Парацетамол ActiFast, paracetamol has a median time to peak plasma concentrations (tmax) of 25 minutes in fasted subjects and 45 minutes in the fed subjects. Maximum plasma concentrations were reached at least twice as fast for Парацетамол ActiFast as for standard paracetamol tablets in both the fed and fasted state (p= 0.0002). Following administration of Парацетамол ActiFast, paracetamol is generally measurable in plasma within 10 minutes in both the fed and fasted state.
Two tablets of Парацетамол ActiFast are required to be taken with 100 ml of water to obtain this fast rate of absorption of paracetamol. The maximum rate of absorption is obtained on an empty stomach. When one tablet is taken the rate of absorption of paracetamol for Парацетамол ActiFast is the same as for standard paracetamol tablets. This is thought to be due to insufficient sodium bicarbonate present in the single tablet dose to increase the rate of paracetamol absorption. In addition, tablets taken with insufficient (<100 mls) water are unlikely to have increased speed of action. (See 5.1 Pharmacodynamic properties).
The extent of absorption of paracetamol from Парацетамол ActiFast tablets is equivalent to that of standard paracetamol tablets as shown by AUC in both fed and fasted states.
Absorption
Paracetamol is rapidly and almost completely absorbed from the gastrointestinal tract. Peak plasma concentrations are reached 30-90 minutes post dose and the plasma half-life is in the range of 1 to 3 hours after therapeutic doses.
Distribution
Drug is widely distributed throughout most body fluids.
Biotransformation
Metabolism occurs almost entirely via hepatic conjugation with glucuronic acid (about 60%), sulphuric acid (about 35%) or cysteine (about 3%). Small amounts of hydroxylated and deacetylated metabolites have also been detected.
Children have less capacity for glucuronidation of the drug than do adults.
In overdosage there is increased N-hydroxylation followed by glutathione conjugation. When the latter is exhausted, reaction with hepatic proteins is increased leading to necrosis.
Elimination
Following therapeutic doses 90-100% of the drug is recovered in the urine within 24 hours.
Pharmacotherapeutic group
The information provided in Pharmacotherapeutic group of Парацетамол
is based on data of another medicine with exactly the same composition as the Парацетамол.
. Be careful and be sure to specify the information on the section Pharmacotherapeutic group in the instructions to the drug Парацетамол directly from the package or from the pharmacist at the pharmacy.
more…
Other Analgesics and Antipyretics (Anilides)
Preclinical safety data
The information provided in Preclinical safety data of Парацетамол
is based on data of another medicine with exactly the same composition as the Парацетамол.
. Be careful and be sure to specify the information on the section Preclinical safety data in the instructions to the drug Парацетамол directly from the package or from the pharmacist at the pharmacy.
more…
Pills; Rectal suppositories; Rectal suppositories for children; Solution for infusion; Substance; Substance-powder; Syrup; Tablets, effervescent
Bolus; Coated tablet; Effervescent tablet; Film-coated tablet; Tablets, soluble
Suspension for ingestion for children
None stated
Preclinical safety data on paracetamol in the literature have not revealed any findings which are of relevance to the recommended dosage and use of the product and which have not been mentioned in other sections of the SmPC.
Mutagenicity
There are no studies relating to the mutagenic potential of Парацетамол Infant Sugar Free Colour Free 120 mg/5 ml Oral Suspension.
In vivo mutagenicity tests of paracetamol in mammals are limited and show conflicting results. Therefore, there is insufficient information to determine whether paracetamol poses a mutagenic risk to man.
Paracetamol has been found to be non-mutagenic in bacterial mutagenicity assays, although a clear clastogenic effect has been observed in mammalian cells in vitro following exposure to paracetamol (3 and 10 mM for 2h).
Carcinogenicity
There are no studies to the carcinogenic potential of Парацетамол Infant Sugar Free Colour Free 120 mg/5 ml Oral Suspension.
There is inadequate evidence to determine the carcinogenic potential of paracetamol in humans. A positive association between the use of paracetamol and cancer of the ureter (but not of other sites in the urinary tract) was observed in a case-control study in which approximate lifetime consumption of paracetamol (whether acute or chronic) was estimated. However, other similar studies have failed to demonstrate a statistically significant association between paracetamol and cancer of the urinary tract, or paracetamol and renal cell carcinoma.
There is limited evidence for the carcinogenicity of paracetamol in experimental animals. Liver cell tumours can be detected in rats following chronic feeding of 500 mg/kg/day paracetamol.
Teratogenicity
There is no information relating to the teratogenic potential of Парацетамол Infant Sugar Free Colour Free 120 mg/5 ml Oral Suspension. In humans, paracetamol crosses the placenta and attains concentrations in the foetal circulation similar to those in the maternal circulation. Intermittent maternal ingestion of therapeutic doses of paracetamol are not associated with teratogenic effects in humans.
Paracetamol has been found to be foetotoxic to cultured rat embryo.
Fertility
A significant decrease in testicular weight was observed when male Sprague-Dawley rats were given daily high doses of paracetamol (500 mg/kg/body weight/day) orally for 70 days.
Incompatibilities
The information provided in Incompatibilities of Парацетамол
is based on data of another medicine with exactly the same composition as the Парацетамол.
. Be careful and be sure to specify the information on the section Incompatibilities in the instructions to the drug Парацетамол directly from the package or from the pharmacist at the pharmacy.
more…
None known
Special precautions for disposal and other handling
The information provided in Special precautions for disposal and other handling of Парацетамол
is based on data of another medicine with exactly the same composition as the Парацетамол.
. Be careful and be sure to specify the information on the section Special precautions for disposal and other handling in the instructions to the drug Парацетамол directly from the package or from the pharmacist at the pharmacy.
more…
No special requirements for disposal.
Парацетамол price
We have no data on the cost of the drug.
However, we will provide data for each active ingredient
The approximate cost of Acetaminophen 500 mg per unit in online pharmacies is from 0.16$ to 0.31$, per package is from 21$ to 31$.
The approximate cost of Acetaminophen 120 mg per unit in online pharmacies is from 2.05$ to 2.05$, per package is from 25$ to 25$.
The approximate cost of Acetaminophen 325 mg per unit in online pharmacies is from 0.21$ to 0.21$, per package is from 21$ to 21$.
The approximate cost of Acetaminophen 650 mg per unit in online pharmacies is from 2.3$ to 2.3$, per package is from 28$ to 28$.
References:
- https://www.drugs.com/search.php?searchterm=ru-paracetamol
- https://pubmed.ncbi.nlm.nih.gov/?term=ru-paracetamol
Available in countries
Find in a country:
When ATH: N02BE01
Title: Paracetamol (Paracetamolum)
Paracetamol: pharmachologic effect
Paracetamol obladaet zharoponizhayushtim, analgesic and moderately anti-inflammatory properties. Inhibits the excitability of the thermoregulatory center, also inhibits (oppresses) synthesis of prostaglandins, inflammatory mediators with a pronounced organic effect.
Paracetamol is rapidly absorbed from the upper intestine, It penetrates into all tissues of the body, metabolised in the liver, with the formation of glucorangide and paracetamol sulfate, excreted mainly by the kidneys. A small amount of paracetamol is deacetylated to form para-aminophenol, which promotes the formation of methemoglobin, this leads to drug toxicity.. The binding of paracetamol to plasma proteins is 25%. The maximum concentration of the drug when administered orally is observed through 30-40 minutes. The antipyretic effect comes through 1,5-2 o’clock. half-life of paracetamol 2-4 o’clock.
With prolonged use of paracetamol in high doses, the drug may have a hepatotoxic effect.
Paracetamol: Indications for use
Paracetamol is indicated for the symptomatic treatment of pain syndrome of various origins of mild to moderate intensity.: headache, toothache, algomenorrhea, myalgia, neuralgia, backache, arthralgia, and the state, which are accompanied by a hyperthermic reaction in infectious and inflammatory diseases.
Paracetamol: application method
Paracetamol tablets use
For adults, a single dose of paracetamol is 0,35-0,5 g 3-4 once a day, maximum single dose for adults 1,5 g, the maximum daily dose 3-4 g. The drug should be taken after meals, drinking plenty of water.
For children 9 to 12 years, The maximum daily dose is 2 g.
For children 3 to 6 years, the maximum daily dose 1-2 g paracetamol, calculated 60 mg 1 kg body weight of the child 3-4 admission.
Paracetamol rectal suppositories application
For children aged from 1 months before 3 years use rectal suppositories, single dose of paracetamol is 15 mg 1 kg body weight, daily – 60 mg 1 kg body weight of the child. Multiplicity of use 3-4 once a day.
For adults and adolescents overweight 60 kg, single dose of 0,35-0,5 g, the maximum single dose 1,5 g 3-4 once a day. Daily dose 3-4 g.
For children 6 to 12 years, The maximum daily dose is 2 g 4 admission.
For children 3 to 6 years, the maximum daily dose 1-2 g paracetamol, calculated 60 mg 1 kg body weight of the child 3-4 admission.
paracetamol syrup application
For children aged from 3 to 12 months 2,5-5 ml syrup (60-120 mg paracetamol).
For children 1 Year to 5 years — 5-10 ml syrup (120-240 mg paracetamol).
For children aged from 5 to 12 years — 10-20 ml syrup (240-480 mg paracetamol).
Adults and children overweight 60 kg – 20-40 ml syrup (480-960 mg paracetamol).
The frequency of taking paracetamol syrup is 3-4 once a day.
If the patient’s condition has not improved while taking paracetamol, it is necessary to inform the doctor about it.
Side effects of paracetamol
Blood system: anemia, thrombocytopenia, agranulocytosis, leukopenia, metgemoglobinemiâ.
From the excretory system: počečnaâ how, aseptic pyuria, glomerulonephritis.
From the nervous system: increased excitability or vice versa drowsiness.
Cardio-vascular system: decreased contractility of the heart muscle.
On the part of the digestive system: nausea, epigastric pain. With prolonged use of paracetamol in high doses, the drug may have a hepatotoxic effect..
Allergic reactions: skin rashes, itch, angioedema.
Hypersensitivity to paracetamol, liver and kidney failure.
When using rectal suppositories, contraindications are inflammatory diseases of the rectal mucosa.
Pregnancy and paracetamol
With caution, paracetamol is prescribed to pregnant women and during lactation..
Paracetamol: drug interaction
With the simultaneous use of barbiturates of antiepileptic drugs, rifampicin may increase the hepatotoxic effect of paracetamol, and also reduces its antipyretic effect. Paracetamol enhances the effect of indirect coagulants (coumarin derivatives). Enhances the action of salicylic acid, caffeine, codeine. When combined with phenobarbital, methemoglobinemia increases. Paracetamol enhances the action of antispasmodics. Do not use paracetamol with other, medicines containing paracetamol, avoid overdose.
Paracetamol: overdose
If the amount of the drug taken is many times higher than the maximum recommended dose, it can cause liver toxicity, accompanied by drowsiness, pale skin and visible mucous membranes, toshnotoy, vomiting and dizziness. Most of these symptoms develop in the first day. If these symptoms appear, you should immediately seek medical help., due to urgent hospitalization. As an antidote use n-acetylcysteine intravenously or orally. Detoxification and symptomatic treatment is also recommended..
Paracetamol: release form
Table. 0,2 g contoured non-cell packing, №10.
Table. 0,2 g strip, №10.
Table. 0.2 g blister, №10.
Paracetamol – 0,2 g.
Table. 325 mg blisters, №6, №12.
Table. 325 mg container, №30.
Paracetamol – 325mg.
Table. 0.5g contoured non-cell packing, №10.
Table. 0,5 g blisters, №10.
Paracetamol – 0,5 g.
Capsules 325 mg blisters, №6, №12.
Capsules 325 mg container №30.
Syrup 125 mg/5ml vial 60 ml, №1.
Syrup 125 mg/5ml vial 100 ml, №1.
Paracetamol – 125mg / 5ml.
Syrup 120 mg/5ml vial 50 ml, №1.
Syrup 120 mg/5ml vial 100 ml, №1.
Syrup 120 mg / 5 ml polymer vial 50 ml, №1.
Syrup 120 mg / 5 ml polymer vial 100 ml, №1.
Syrup 120 mg / 5 ml polymer jar 100 ml, №1.
Paracetamol — 120 mg / 5ml.
Rectal Suppositories 0,08 g strip, №10.
Paracetamol – 0,08 g.
Rectal Suppositories 0,17 g strip, №10.
Paracetamol – 0,17 g.
Rectal Suppositories 0,33 g strip, №10.
Paracetamol – 0,33 g.
Suspension 120 mg / 5 ml vial 100 ml.
Suspension 120 mg/5 ml polyethylene container 200 ml.
Paracetamol – 120 mg / 5 ml.
Paracetamol: storage conditions
Keep out of the reach of children at an air temperature not exceeding 25 degrees Celsius.
Paracetamol Synonyms
Panadol Junior, Tylenol, Ifimol, Aminodol Acetophene, Panadol, Panadol solubl, Opradol, Ushamol, Valadol, Valorin, Acelifen, abesanil, Acetalgin, Actazole, Algotoropil, Aminophen, Dimindol, Dolanex, Dolipram,Apagan, Biocetamol, Celifen, Cetadol, Dapirex, Dolamine, Efferalglan, Erocetamol, Febridol, Pacimol, Pyrenees, Tralgon, Pyremol, Febricet, Calpol, Febrinol, Nasprin, Acemol, Cetanil, Apamide, Acetaminophen, Acetaminophenol, Hemcetafen, Datril, Dexamol, Effered, Febrinil, Fendon, , Myalgin, Napamol, Naprinol, Alvedon, Amphenol, Repeated, Deminofen, Mexican, Apanol, Nizacetol, Rolocin, Tempramol, Volpan, Vinadol, Akamol, Bindard, Paramol, Metamol, I wish, Tilenol, Valgesic, Minoset.
See also the list of analogues of the drug Paracetamol.
Paracetamol: pharmacological group
Painkillers and anti-inflammatory drugs
Non-narcotic analgesics, antipyretic, nonsteroidal anti-inflammatory and antirheumatic drugs. Analgesics-antipyretics.
Active substance: Paracetamol
Paracetamol: additionally
Paracetamol is part of such combination drugs: Paravit for children, Paramin, Parapasta, Para-trawl, Parafex, Pentalgin, Pharmacitron, Fervex, Coldrex, Cold Flu, Ascophene, Tempalgin, Sedalgin-neo, Sedal-M.
Paracetamol interaction
| Active substance | Description of interaction |
| Glibenclamide | FMR: synergism. Against the background of the effect of paracetamol is enhanced. |
| Metoclopramide | FKV. It accelerates absorption. |
| Ethanol | Against the background of paracetamol should not be taken Alcoholic drinks. |


