E-mail (используется для входа) *
Краткая информация о себе, как специалисте (специализация, ученая степень и т.п.)
Аппарат и направления УЗИ, с которыми Вы работаете
На каком ультразвуковом аппарате Вы работаете?
добавить аппарат
С какими направлениями ультразвуковой диагностики вы работаете?
| Отметить все / снять все | |||
| Акушерство | Абдоминальные исследования | ||
| Гинекология | Сердечно-сосудистые исследования | ||
| Маммология | Мускуло-скелетные исследования | ||
| Урология | Поверхностно-расположенные органы | ||
| Педиатрия | Другие направления |
Главная » Philips » Руководство пользователя ультразвуковой системы Philips Epiq 5
Содержание скрывать
1
Руководство пользователя ультразвуковой системы Philips Epiq 5
2
Похожие сообщения
Руководство пользователя ультразвуковой системы Philips Epiq 5
View Fullscreen
Похожие сообщения
-
PHILIPS Soundbar Руководство пользователя
Soundbar
-
Руководство пользователя Philips серии HD9630
Philips HD9630 Series User Manual — Download [оптимизированное] Philips HD9630 Series User Manual — Download
-
Руководство пользователя игрового монитора Philips
Руководство пользователя игрового монитора Philips — Оптимизированный PDF-файл Руководство пользователя игрового монитора Philips — Исходный PDF-файл
-
Руководство пользователя Philips Roku TV
Руководство пользователя Philips Roku TV — Оптимизированный PDF-файл Руководство пользователя Philips Roku TV — Исходный PDF-файл
Оставить комментарий
Ваш электронный адрес не будет опубликован. Обязательные поля помечены * *
КОММЕНТАРИЙ *
Имя и фамилия
Эл. адрес
Cайт
Сохраните мое имя, адрес электронной почты и веб-сайт в этом браузере для следующего комментария.
Методы получения и анализа данных в ультразвуковой диагностике
Ознакомьтесь с самыми современными методами получения и анализа данных
Ознакомьтесь с самыми современными методами с помощью пошаговых руководств. Эти методы помогут вам более точно и эффективно получать и анализировать данные. Эти онлайн-ресурсы помогут вам быстро и без труда освоить методы ультразвуковых исследований в удобном для вас месте и именно тогда, когда вам будет это необходимо.
Как выполнить полнообъемный сбор данных
EPIQ
Пошаговое руководство по полнообъемному сбору данных на ультразвуковой системе Philips EPIQ. Этот режим используется при необходимости в получении большого объема данных. Данное видео иллюстрирует сбор данных для всего сердца.
Как выполнять сбор 4D-данных
EPIQ
Пошаговое руководство по сбору 4D-данных на ультразвуковой системе Philips EPIQ.
Как выполнять эластографию сдвиговой волной
EPIQ
Пошаговое руководство по использованию метода эластографии сдвиговой волной для исследования печени с помощью ультразвуковой системы Philips EPIQ. Эластография сдвиговой волной применяется для оценки жесткости тканей печени у пациентов, страдающих циррозом или фиброзом печени.
Как выполнять деформационную эластографию
EPIQ
Пошаговое руководство по проведению деформационной эластографии с помощью ультразвуковой системы Philips EPIQ. Деформационная эластография — это неинвазивный метод визуализации в реальном времени, позволяющий получать и накладывать на серошкальное 2D-изображение слой с информацией, характеризующей жесткость тканей. Этот метод можно использовать при исследовании молочных желез для оценки жесткости поражения, а также при ультразвуковом исследовании области шеи и опорно-двигательного аппарата.
EPIQ и Affiniti | Редактор шаблонов отчетов
Как использовать редактор шаблонов отчетов на ПК и ультразвуковой системе EPIQ или Affiniti.
EPIQ и Affiniti | Беспроводное подключение к сети
Как установить беспроводное соединение между ПК и ультразвуковой системой EPIQ или Affiniti.
EPIQ и Affiniti | Прямое подключение к сети
Как установить прямое соединение между ПК и ультразвуковой системой EPIQ или Affiniti с помощью Ethernet-кабеля.
DICOM-кодирование
Как кодировать результаты пользовательских измерений и расчетов для создания DICOM-отчета.

ClearVue 850 | Stress Echo
Как выполнить стресс-эхокардиографическое исследование на ультразвуковой системе ClearVue 850.
ClearVue 850 | Датчик L12-5 38

Обзор нового датчика L12-5 38 и доступных предустановок.
ClearVue 850 | QLAB 10.4
Как использовать полнообъемный набор данных для сердца в приложении 3D Quantification Advanced (3DQA).

Как выполнить количественный 3D-анализ с помощью приложения 3DQA
QLAB 10
Пошаговое руководство по использованию полнообъемного набора данных для сердца в приложении 3D Quantification Advanced (3DQA) на ультразвуковой системе Philips EPIQ. 3DQA является средством полуавтоматического определения границ, которое используется с 3D-изображений сердца в пакете QLAB.
QLAB 10 | 3D Quantification (3DQ)
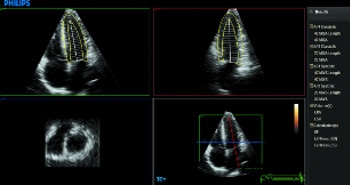
Как выполнить расчет фракции выброса по двум проекциям.
QLAB 10 | 3D Quantification Advanced (3DQA)
Как использовать полнообъемный набор данных для сердца в приложении 3D Quantification Advanced (3DQA).
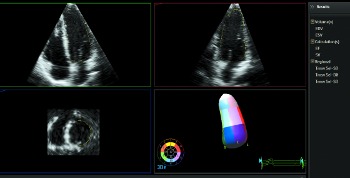
QLAB 10 | HeartModelA.I.

Как использовать приложение HeartModelA.I. (доступно только на системах EPIQ 2.0) и с помощью технологии Anatomical Intelligence провести количественный анализ левого желудочка и левого предсердия.
QLAB 10 | aCMQA.I.
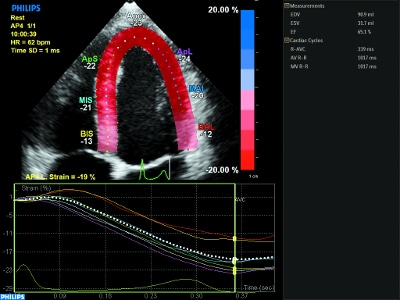
Как использовать приложение aCMQA.I. для расчета фракции выброса в режиме 2D, объемов и пиковой систолической деформации с помощью технологии отслеживания спекл-шума.
Вы покидаете официальный веб-сайт Philips Здравоохранение (“Philips”). Любые ссылки на сторонние веб-сайты, которые могут быть размещены на этом сайте, предоставлены исключительно для вашего удобства. Philips не даёт никаких гарантий относительно каких-либо сторонних веб-сайтов и содержащейся на них информации.
Я понимаю
You are about to visit a Philips global content page
Continue
Have a look at the manual Philips Epiq 5 Manual online for free. It’s possible to download the document as PDF or print. UserManuals.tech offer 619 Philips manuals and user’s guides for free. Share the user manual or guide on Facebook, Twitter or Google+.

DICOM Conformance Statement EPIQ 7 and 5 Release 1.5.x.x Affiniti 70 and 50 Release 1.5.x.x 000490000000111 Rev A 24 Nov 2015 © Koninklijke Philips Electronics N.V. 2015 All rights are reserved.

0.1 Revision History Document Version Date of Issue Author s Description A 24 Nov 2015 EC, JL, ML Initial Release E7 and E5/A70 and A50 1.5.x.x DICOM Conformance Statement 000490000000111 Rev A Page 2

1 CONFORMANCE STATEMENT OVERVIEW Table 1.1 provides an overview of the supported network services. Table 1.1 NETWORK SERVICES Networking SOP Classes User of Service (SCU) Provider of Service (SCP) Transfer Ultrasound Image Storage Yes* Yes* Ultrasound Image Storage (Retired) Yes* Yes* Ultrasound Multiframe Image Storage Yes* Yes* Ultrasound Multiframe Image Storage (Retired) Yes* Yes* Secondary Capture Image Storage Yes* Yes* Multi-frame True Color Secondary Capture Image Storage Yes* Yes* CT Image Storage Yes* Yes* Enhanced CT Image Storage Yes* Yes* Digital Mammography X -Ray Image Storage – For Presentation Yes* Yes* Digital Mammography X -Ray Image Storage – For Processing Yes* Yes* MR Image Storage Yes* Yes* Enhanced MR Image Storage Yes* Yes* MR Spectroscopy Image Storage Yes* Yes* Multi-frame Single Bit Secondary Capture Image Storage Yes* Yes* Multi-frame Grayscale Byte Secondary Capture Image Storage Yes* Yes* Multi-frame Grayscale Word Secondary Capture Image Storage Yes* Yes* Positron Emission Tomography Image Storage Yes* Yes* X-Ray Angiographic Image Storage Yes* Yes* Private 3D Presentation State Storage Yes* Yes* Comprehensive SR Storage Yes* No Storage Commitment Push Model Yes* No Query/Retrieve Study Root Query/Retrieve -FIND Yes** No Study Root Query/Retrieve -MOVE Yes** No Workflow Management E7 and E5/A70 and A50 1.5.x.x DICOM Conformance Statement 000490000000111 Rev A Page 3

The Philips EPIQ and Affiniti Ultrasound system s implement the necessary DICOM
® services to download
worklists from an information system, store I mages and Structured Reports to a network storage device,
commit previously stored US images, store Images and Structured Reports to CD , DVD , or removable USB
Media s torage device s, print to a networked DICOM printer device , receive previously stored ultrasound and
other modality images, and inform the information system about the work actually done.
* Purchasable option “Netlink DICOM 3.0”. DICOM Printing does not require an option.
** Requires option “ Ultrasound Query Retrieve” or Multi -Modality Query Retrieve.
The SOP Classes are categorized as shown in Table 1.2 :
Table 1.2
UID VALUES
UID Value UID NAME Category
1.2.840.10008.1.20.1 Storage Commitment
Push Model SOP Class Workflow Management
1.2.840.10008.3.1.2.3.3 Modality Performed
Procedure Step SOP
Class Workflow Management
1.2.840.10008.5.1.1.9 Basic Grayscale Print
Management Meta SOP
Class Print Management
1.2.840.10008.5.1.1.18 Basic Color Print
Management Meta SOP
Class Print
Management
1.2.840.10008.5.1.4.1.1.1.2 Digital Mammography X -
Ray Image Storage – For
Presentation Transfer
1.2.840.10008.5.1.4.1.1.1.2.1 Digital Mammography X -
Ray Image Storage – For
Processing Transfer
1.2.840.10008.5.1.4.1.1.2 CT Image Storage Transfer
1.2.840.10008.5.1.4.1.1.2.1 Enhanced CT Image
Storage Transfer
1.2.840.10008.5.1.4.1.1.3.1 Ultrasound Multi -frame
Image Storage Transfer
® DICOM is the registered trademark of the National Electrical Manufacturers Association for its standards publications relating to digital
communications of medical information.
Modality Worklist Information Model - FIND Yes* No
Modality Performed Procedure Step Yes* No
Print Management
Basic Grayscale Print Management Yes No
Basic Color Print Management Yes No
E7 and E5/A70 and A50 1.5.x.x DICOM Conformance Statement 000490000000111 Rev A Page 4

UID Value UID NAME Category 1.2.840.10008.5.1.4.1.1.3 Ultrasound Multi -frame Image Storage (Retired) Transfer 1.2.840.10008.5.1.4.1.1.4 MR Image Storage Transfer 1.2.840.10008.5.1.4.1.1.4.1 Enhanced MR Image Storage Transfer 1.2.840.10008.5.1.4.1.1.4.2 MR Spectroscopy Storage Transfer 1.2.840.10008.5.1.4.1.1.6.1 Ultrasound Image Storage Transfer 1.2.840.10008.5.1.4.1.1.6 Ultrasound Image Storage (Retired) Transfer 1.2.840.10008.5.1.4.1.1.7 Secondary Capture Image Storage Transfer 1.2.840.10008.5.1.4.1.1.7.1 Multi-frame Single Bit Secondary Capture Image Storage Transfer 1.2.840.10008.5.1.4.1.1.7.2 Multi-frame Grayscale Byte Secondary Capture Image Storage Transfer 1.2.840.10008.5.1.4.1.1.7.3 Multi-frame Grayscale Word Secondary Capture Image Storage Transfer 1.2.840.10008.5.1.4.1.1.7.4 Multi-frame True Color Secondary Capture Image Storage Transfer 1.2.840.10008.5.1.4.1.1.128 Positron Emission Tomography Image Storage Transfer 1.2.840.10008.5.1.4.1.1.12.1 X-Ray Angiographic Image Storage Transfer 1.3.46.670589.2.5.1.1 Private 3D Presentation State Storage Transfer 1.2.840.10008.5.1.4.1.1.88.33 Comprehensive SR Storage Transfer 1.2.840.10008.5.1.4.1.2.2.1 Study Root Query/Retrieve Information Model – FIND Query/Retrieve 1.2.840.10008.5.1.4.1.2.2. 2 Study Root Query/Retrieve Information Model – MOVE Query/Retrieve 1.2.840.10008.5.1.4.31 Modality Worklist Information Model – FIND Workflow Management 1.2.840.10008.5.1.4.31 Modality Worklist Information Model – FIND Workflow Management Table 1.3 specifies the Media Storage Application Profiles supported. E7 and E5/A70 and A50 1.5.x.x DICOM Conformance Statement 000490000000111 Rev A Page 5

Table 1.3 MEDIA SERVICES Media Storage Application Profile Write Files (FSC or FSU) Read Files (FSR) STD-US -SC -SF&MF -CDR Yes / Yes Yes(1) STD-US -SC -SF&MF -DVD Yes / Yes Yes(1) STD-GEN -USB- JPEG Yes / Yes Yes(1) (1) Structured Reports cannot be imported. E7 and E5/A70 and A50 1.5.x.x DICOM Conformance Statement 000490000000111 Rev A Page 6

2 TABLE OF CONTENTS 1 CONFORMANCE STATEMENT OVERVIEW .......................................................................................... 3 2 TABLE OF CONTENTS ........................................................................................................................... 7 3 INTRODUCTION .................................................................................................................................... 11 3.1 AUDIENCE ............................................................................................................................. 11 3.2 REMARKS .............................................................................................................................. 11 3.3 DEFINITIONS, TERMS AND ABBREVIATIONS ................................................................... 11 3.4 REFERENCES ....................................................................................................................... 12 4 NETWORKING ....................................................................................................................................... 14 4.1 IMPLEMENTATION MODEL.................................................................................................. 14 4.1.1 Application Data Flow ................................................................................................... 14 4.1.2 Functional Definition of AEs ......................................................................................... 16 4.1.2.1 Functional Definition of Storage Application Entity ............................................. 16 4.1.2.2 Functional Definition of Workflow Application Entity .......................................... 17 4.1.2.3 Functional Definition of Hardcopy Application Entity .......................................... 17 4.1.3 Sequencing of Real-World Act ivities ............................................................................ 18 4.2 AE SPECIFICATIONS ............................................................................................................ 21 4.2.1 Storage Application Entity Specification ....................................................................... 21 4.2.1.1 SOP Classes ...................................................................................................... 21 4.2.1.2 Association Establishment Policy ....................................................................... 22 4.2.1.2.1 General ..................................................................................... 22 4.2.1.2.2 Number of Associations ............................................................ 22 4.2.1.2.3 Asynchronous Nature ............................................................... 22 4.2.1.2.4 Implementation Identifying Information..................................... 22 4.2.1.3 Association Initiation Policy ................................................................................ 23 4.2.1.3.1 Activity – Store Images and Structured Reports ...................... 23 4.2.1.3.1.1 Description and Sequencing of Activities ...................................... 23 4.2.1.3.1.2 Proposed Presentation Contexts ................................................... 25 4.2.1.3.1.3 SOP Specific Conformance ........................................................... 27 4.2.1.3.1.3.1 Image and Comprehensive Structured Report Storage SOP Classes 27 4.2.1.3.1.3.2 SOP Specific Conformance for Storage Commitment Push Model SOP Class ...................................................................................... 27 4.2.1.3.2 Activity – Query and Request Retrieval of Studies ................... 29 4.2.1.3.2.1 Description and Sequenci ng of Activities ...................................... 29 4.2.1.3.2.2 Proposed Presentation Contexts ................................................... 30 4.2.1.3.2.3 SOP Specific Conformance for SOP Classes ............................... 30 4.2.1.3.2.3.1 Study Root Query Retrieve – FIND SOP Class ................ 30 4.2.1.3.2.3.2 Study Root Query Retrieve – MOVE SOP Class ............. 31 4.2.1.4 Association Acceptance Policy ........................................................................... 31 4.2.1.4.1 Activity – Receive Storage Commitment Response ................. 31 4.2.1.4.1.1 Description and Sequencing of Activities ...................................... 31 4.2.1.4.1.2 Accepted Presentation Contexts ................................................... 31 4.2.1.4.1.3 SOP Specific Conformance for Storage Commitment Push Model SOP Class 32 4.2.1.4.1.3.1 Storage Commitment Notifications (N -EVENT -REPORT) 32 4.2.1.4.1.3.2 Storage Commitment Attributes (N -EVENT -REPORT) .... 32 4.2.1.4.2 Activity – Storage from a Remote Storage SCU ...................... 33 4.2.1.4.2.1 Description and Sequencing of Activities ...................................... 33 4.2.1.4.2.2 Accepted Presentation Contexts ................................................... 34 4.2.2 Workflow Application Entity Specification ..................................................................... 36 E7 and E5/A70 and A50 1.5.x.x DICOM Conformance Statement 000490000000111 Rev A Page 7

4.2.2.1 SOP Classes ...................................................................................................... 36 4.2.2.2 Association Establishment Policy ....................................................................... 36 4.2.2.2.1 General ..................................................................................... 36 4.2.2.2.2 Number of Associations ............................................................ 36 4.2.2.2.3 Asynchronous Nature ............................................................... 36 4.2.2.2.4 Implementation Identifying Information..................................... 36 4.2.2.3 Association Initiation Policy ................................................................................ 37 4.2.2.3.1 Activity – W orklist Update ......................................................... 37 4.2.2.3.1.1 Description and Sequencing of Activities ...................................... 37 4.2.2.3.1.1.1 Worklist Update using Broad Query ................................. 37 4.2.2.3.1.1.2 Worklist Update using Patient Query ................................ 38 4.2.2.3.1.2 Proposed Presentation Contexts ................................................... 38 4.2.2.3.1.3 SOP Specific Conformance for Modality Worklist ......................... 38 4.2.2.3.2 Activity –Acquire Images .......................................................... 41 4.2.2.3.2.1 Description and Sequencing of Activities ...................................... 41 4.2.2.3.2.2 Proposed Presentation Contexts ................................................... 43 4.2.2.3.2.3 SOP Specific Conformance for MPPS .......................................... 43 4.2.2.4 Association Acceptance Policy ........................................................................... 46 4.2.3 Hardcopy Application Entity Specification .................................................................... 46 4.2.3.1 SOP Classes ...................................................................................................... 46 4.2.3.2 Association Establishment Policy ....................................................................... 46 4.2.3.2.1 General ..................................................................................... 46 4.2.3.2.2 Number of Associations ............................................................ 46 4.2.3.2.3 Asynchronous Nature ............................................................... 47 4.2.3.2.4 Implementation Identifying Information..................................... 47 4.2.3.3 Association Initiation Policy ................................................................................ 47 4.2.3.3.1 Activity – Film Images ............................................................... 47 4.2.3.3.1.1 Description and Sequencing of Activities ...................................... 47 4.2.3.3.1.2 Proposed Presentation Contexts ................................................... 48 4.2.3.3.1.3 SOP Specific Conformance for all Print SOP Classes .................. 49 4.2.3.3.1.3.1 Printer SOP Class Operations (N -GET) ........................... 49 4.2.3.3.1.3.2 Printer SOP Class Notifications (N -EVENT -REPORT) .... 50 4.2.3.3.1.3.3 SOP Specific Conformance for the Film Session SOP Class 50 4.2.3.3.1.3.4 Film Session SOP Class Operations (N -CREATE) .......... 50 4.2.3.3.1.4 SOP Specific Conformance for the Film Box SOP Class .............. 51 4.2.3.3.1.4.1 Film Box SOP Class Operations (N -CREATE) ................. 51 4.2.3.3.1.4.2 Film Box SOP Class Operations (N -ACTION) .................. 53 4.2.3.3.1.5 SOP Specific Conformance for the Image Box SOP Class ........... 53 4.2.3.3.1.5.1 Image Box SOP Class Operations (N -SET) ..................... 53 4.2.3.4 Association Acceptance Policy ........................................................................... 54 4.2.4 Verification Application Entity specification .................................................................. 54 4.2.4.1 SOP Class .......................................................................................................... 54 4.2.4.2 Association Establishment Policy ....................................................................... 54 4.2.4.2.1 General ..................................................................................... 54 4.2.4.2.2 Number of Associations ............................................................ 55 4.2.4.2.3 Asynchronous Nature ............................................................... 55 4.2.4.2.4 Implementation Identifying Information..................................... 55 4.2.4.3 Association Initiation Policy ................................................................................ 56 4.2.4.3.1 Activity – Verify Remote SCP ................................................... 56 4.2.4.3.1.1 Description and Sequencing of Activities ...................................... 56 4.2.4.3.1.2 Proposed Presentation Contexts ................................................... 56 4.2.4.3.1.3 SOP Specific Conformance for Verification ................................... 57 4.2.4.4 Association Acceptance Policy ........................................................................... 58 4.2.4.4.1 Activity – Verification By Remote AE ........................................ 58 4.2.4.4.1.1 Description and Sequencing of Activities ...................................... 58 E7 and E5/A70 and A50 1.5.x.x DICOM Conformance Statement 000490000000111 Rev A Page 8

4.2.4.4.1.2 Accepted Presentation Contexts ................................................... 59 4.2.4.4.1.3 SOP Specific Conformance for Verification ................................... 59 4.3 NETWORK INTERFACES ..................................................................................................... 59 4.3.1 Physical Network Interface ........................................................................................... 59 4.3.2 Additional Protocols ...................................................................................................... 60 4.3.3 IPv4 and IPv6 Support .................................................................................................. 60 4.4 CONFIGURATION ................................................................................................................. 60 4.4.1 AE Title/Presentation Address Mapping ....................................................................... 60 4.4.1.1 Local AE Title ...................................................................................................... 60 4.4.1.2 Remote AE Title/Presentation Address Mapping ............................................... 61 4.4.1.2.1 Image Storage Configuration .................................................... 61 4.4.1.2.2 Structured Report Storage Configuration ................................. 62 4.4.1.2.3 Advanced DICOM Device Association Configuration ............... 62 4.4.1.2.4 Serial Structured Report Storage Configuration ....................... 63 4.4.1.2.5 Workflow Configuration ............................................................ 63 4.4.1.2.6 Hardcopy .................................................................................. 63 4.4.2 Parameters ................................................................................................................... 63 5 MEDIA STORAGE .................................................................................................................................. 64 5.1 IMPLEMENTATION MODEL.................................................................................................. 64 5.1.1 Application Data Flow ................................................................................................... 64 5.1.2 Functional Definition of AEs ......................................................................................... 65 5.1.2.1 Functional Definition of Media Application Entity ............................................... 65 5.1.3 Sequencing of Real-World Activities ............................................................................ 65 5.1.4 File Meta Information Options ...................................................................................... 65 5.2 AE SPECIFICATIONS ............................................................................................................ 65 5.2.1 Media Application Entity Specification .......................................................................... 65 5.2.1.1 File Meta Information for the Application Entity .................................................. 65 5.2.1.2 Real-World Activities ........................................................................................... 66 5.2.1.2.1 Activity – Export to Media ......................................................... 66 5.2.1.2.2 Activity – Import from Media ..................................................... 66 5.2.1.2.3 Activity – Update to Media ........................................................ 66 5.2.1.2.3.1 Media Storage Application Profiles ................................................ 66 5.2.1.2.3.2 Options .......................................................................................... 66 6 TRANSFORMATION OF DICOM TO CDA ............................................................................................ 68 7 SUPPORT OF CHARACTER SETS ...................................................................................................... 68 8 SECURITY .............................................................................................................................................. 68 8.1 GENERAL SECURITY ........................................................................................................... 68 8.2 SUPPORTED DICOM SECURITY PROFILES ...................................................................... 68 8.2.1 TLS Secure Transport Connection Profiles .................................................................. 68 9 ANNEXES ............................................................................................................................................... 70 9.1 IOD CONTENTS .................................................................................................................... 70 9.1.1 Created SOP Instances ................................................................................................ 70 9.1.1.1 US or US Multiframe Image IOD ........................................................................ 70 9.1.1.2 Secondary Capture IOD ..................................................................................... 71 9.1.1.3 Multi-Frame True Color Secondary Capture IOD ............................................... 71 9.1.1.4 Comprehensive Structured Report IOD .............................................................. 72 9.1.1.5 Attribute Content by Module ............................................................................... 72 9.1.1.5.1 Common Modules ..................................................................... 72 9.1.1.5.2 US or Multiframe Image Modules ............................................. 76 9.1.1.5.3 Secondary Capture Image Modules ......................................... 82 9.1.1.5.4 Comprehensive Structured Report Modules ............................ 84 9.1.2 Usage of Attributes from Received IOD’s ..................................................................... 87 9.1.3 Attribute Mapping ......................................................................................................... 87 E7 and E5/A70 and A50 1.5.x.x DICOM Conformance Statement 000490000000111 Rev A Page 9

9.1.4 Coerced/Modified Fields ............................................................................................... 88 9.1.5 Attribute Anonymization ................................................................................................ 89 9.2 DATA DICTIONARY OF PRIVATE ATTRIBUTES ................................................................ 90 9.3 CODED TERMINOLOGY AND TEMPLATES ........................................................................ 90 9.4 GRAYSCALE IMAGE CONSISTENCY .................................................................................. 91 9.5 STANDARD EXTENDED/SPECIALIZED/PRIVATE SO P CLASSES ................................... 91 9.5.1 Ultrasound Image and Multi -frame Image SOP Classes ............................................. 91 9.5.2 3D Presentation State Private SOP Class ................................................................... 92 9.6 PRIVATE TRANSFER SYNTAXES ....................................................................................... 92 A.1 STRUCTURED REPORTS .................................................................................................... 93 A.1.1 Introduction ................................................................................................................... 93 A.1.1.1 Measurements Linked to Images ....................................................................... 93 A.1.2 Clinical Scope ............................................................................................................... 94 A.2 APPLICATIONS THAT EXPORT STRUCTURED REPO RTS FROM EPIQ AND AFFINITI . 94 A.3 DICOM STRUCTURED REP ORT EXPORT SPECIFICATIONS .......................................... 94 A.3.1 Philips Healthcare Ultrasound Data Portal Website ..................................................... 94 A.4 PRIVATE TEMPLATE EXTENSIONS .................................................................................... 95 A.4.1 TID5001: OB-GYN Patient Characteristics .................................................................. 95 A.4.2 TID5008: Fetal Biometry Group ................................................................................... 95 A.4.3 TID5101: Vascular Patient Characteristics ................................................................... 96 A.4.4 TID5202: Echocardiography Patient Characteristics ................................................... 97 A.5 USER-DEFINED MEASUREMENTS AND CALCULATIONS ............................................... 98 A.5.1 Description .................................................................................................................... 98 A.5.1.1 Philips Generic DICOM Export Format ............................................................... 98 A.5.1.2 DICOM Template Compatible Export Format ..................................................... 98 A.5.1.3 Private Template and Template Extensions ....................................................... 99 A.5.1.3.1 TID5000: OB-GYN Ultrasound Procedure Report .................... 99 A.5.1.3.2 TID5100: Vascular Ultrasound Report ...................................... 99 A.5.1.3.3 TID5200: Echocardiography Procedure Report ....................... 99 A.5.1.3.4 TID995300: Pediatric Echocardiography Procedure Report .... 99 A.5.1.3.5 TID9900: User -defined concepts .............................................. 99 A.5.1.3.6 TID9901: User-defined concept ................................................ 99 A.5.1.3.7 TID9902: Fetal Heart Section ................................................. 100 A.5.1.3.8 TID 5009: Fetal Biophysical Profile Section ........................... 100 A.5.1.3.9 TID 5016: Pelvis and Uterus Section ...................................... 101 B.1 BULK PRIVATE ATTRIBU TES ............................................................................................ 102 E7 and E5/A70 and A50 1.5.x.x DICOM Conformance Statement 000490000000111 Rev A Page 10
All Philips manuals
Comments (0)
Related Manuals for Philips Epiq 5 Manual
Similar to Philips HD3 Manuals, User Guides and Instructions:
-
Fisher & Paykel Oracle 452
Multi Patient Instructions for UseORAL MASK 452185043485 RevBFISHER & PAYKEL HEALTHCARE INTERNATIONAL PO Box 14 348, Panmure, Auckland 1134, New ZealandTel: +64 9 574 0100Fax: +64 9 574 0158Email: [email protected]: www.fphcare.comUSA Tel: 1800 446 3908or +1 949 470 3900Fax: +1 949 470 3933AUSTRALIA Tel: + …
Oracle 452 Medical Equipment, 2
-
Gima 33363
SILVER/SILVER CLHORIDE REUSABLE ECG ELECTRODES AND ACCESSORIESDIRECTIONS FOR USEINDICATIONS:Surface ECG recording.PRELIMINARY NOTESNormally a surface ECG (at rest or during stress) consists of 12 leads: 3 limb bipolar recordings, 3 limbunipolar recordings and 6 precordial unipolar recordings. Therefore, 10 electrodes, …
33363 Medical Equipment, 2
-
Alaxo AlaxoStent C
AlaxoStentAP004 AlaxoStent [email protected] DAGEBRAUCHSANWEISUNG DEINSTRUCTIONS FOR USE ENMANUAL DE INSTRUCCIONES ESKÄYTTÖOHJEET FIMANUEL D´UTILISATION FRΟΔΗΓΊΕΣ ΧΡΗΣΗΣ GRUPUTE ZA UPOTREBU HRHASZNÁLATI ÚTMUTATÓ HU ISTRUZIONI PER L´USO ITGEBRUIKSAAN …
AlaxoStent C Medical Equipment, 56
-
Care Fusion Snowden-Pencer
Proofed by: _________________________________ Date: ______________Dimensions checked: _______________ Copy checked: __________________36-0151B10-16-18David KnuthLaparoscopic Ergonomic Take-Apart InstrumentsInstruments laparoscopiques ergonomiquesdémontablesLaparoskopische und ergonomische zerlegbareInstrumenteStrument …
Snowden-Pencer Medical Equipment, 149
-
Cardinal Health NPWT PRO
Cardinal Health™ PRO/PRO to GONegative Pressure Wound Therapy Clinician Quick Reference Guide ON/MUTE ButtonOFF ButtonUP ButtonDOWN ButtonCanister Release ButtonContinuous / Intermittent ModeBattery Charging PortCanister HolderPressure SettingAlert DisplayPower / Charging Status NOTE: Please refer to the Cardinal Hea …
NPWT PRO Medical Equipment, 2




